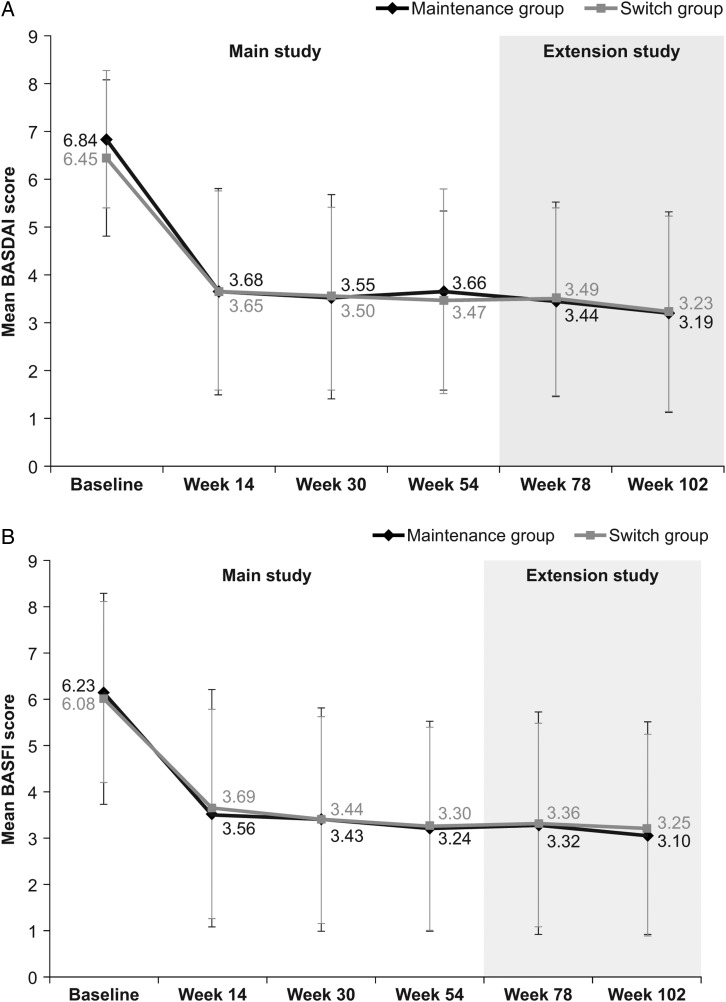
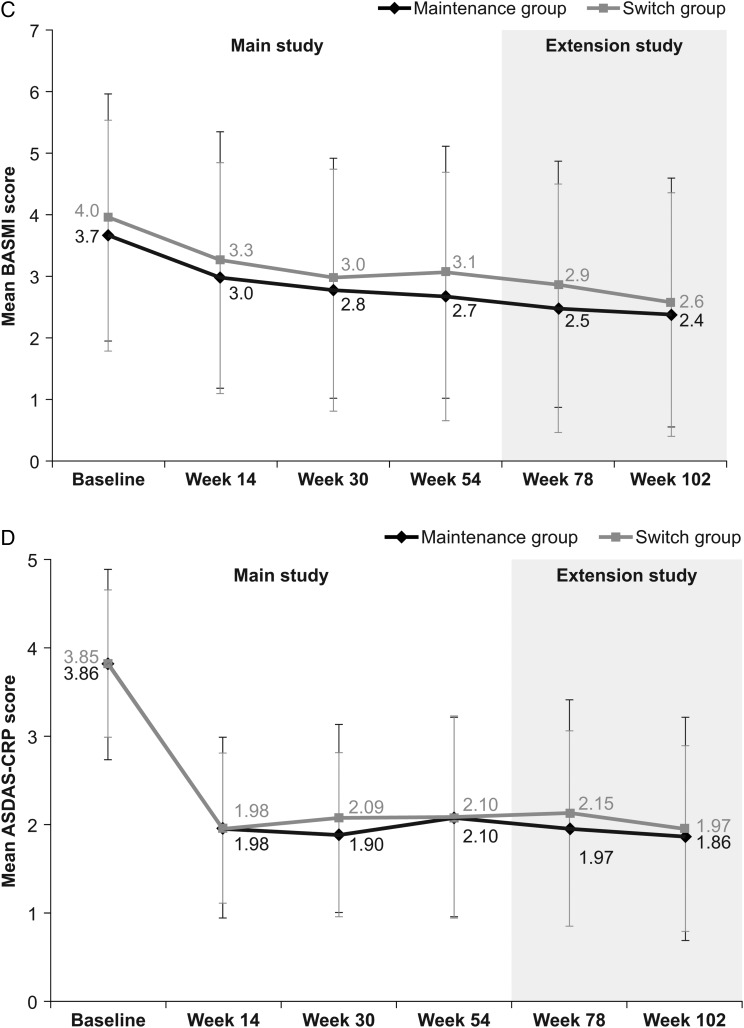
Figure 2.
Additional efficacy end points assessed in the PLANETAS extension study. Mean (SD) BASDAI (A), BASFI (B), BASMI (C) and ASDAS-CRP (D) scores in maintenance* (n=88) and switch** (n=86) groups during the main study and the extension study (efficacy population). *Patients treated with CT-P13 during the 54 weeks of the main study and the 48-week extension study. **Patients treated with RP during the 54 weeks of the main study and then switched to CT-P13 during the 48-week extension study. ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CRP, C reactive protein; RP, reference product.