Abstract
Background
In 2012, a European initiative called Single Hub and Access point for pediatric Rheumatology in Europe (SHARE) was launched to optimise and disseminate diagnostic and management regimens in Europe for children and young adults with rheumatic diseases. Juvenile dermatomyositis (JDM) is a rare disease within the group of paediatric rheumatic diseases (PRDs) and can lead to significant morbidity. Evidence-based guidelines are sparse and management is mostly based on physicians' experience. Consequently, treatment regimens differ throughout Europe.
Objectives
To provide recommendations for diagnosis and treatment of JDM.
Methods
Recommendations were developed by an evidence-informed consensus process using the European League Against Rheumatism standard operating procedures. A committee was constituted, consisting of 19 experienced paediatric rheumatologists and 2 experts in paediatric exercise physiology and physical therapy, mainly from Europe. Recommendations derived from a validated systematic literature review were evaluated by an online survey and subsequently discussed at two consensus meetings using nominal group technique. Recommendations were accepted if >80% agreement was reached.
Results
In total, 7 overarching principles, 33 recommendations on diagnosis and 19 recommendations on therapy were accepted with >80% agreement among experts. Topics covered include assessment of skin, muscle and major organ involvement and suggested treatment pathways.
Conclusions
The SHARE initiative aims to identify best practices for treatment of patients suffering from PRD. Within this remit, recommendations for the diagnosis and treatment of JDM have been formulated by an evidence-informed consensus process to produce a standard of care for patients with JDM throughout Europe.
Keywords: Autoimmune Diseases, Dermatomyositis, Treatment
Introduction
In 2012, Single Hub and Access point for pediatric Rheumatology in Europe (SHARE) was launched with the aim of optimising and disseminating diagnostic and management regimens for children and young people with rheumatic diseases. This includes juvenile dermatomyositis (JDM); the focus of this paper. Clear recommendations can help clinicians in the care of patients with JDM as no international consensus regarding diagnosis and treatment is currently available and management therefore varies.
Methods
A committee of 19 experts in paediatric rheumatology, 2 experts in exercise physiology and physical therapy was established to develop recommendations for JDM based on consensus, but evidence informed, using the European League Against Rheumatism (EULAR) standard operating procedures for developing best practice.1 2
Systematic literature search
The electronic databases PubMed/MEDLINE, Embase and Cochrane were searched twice for eligible articles in June 2013 and subsequently in February 2015. All synonyms of JDM were searched in MeSH/Emtree terms, title and abstract. Reference tracking was performed in all included studies (full search strategy in online supplementary figure S1). Experts (FBE, LJMC, AvR-K) selected papers relevant to JDM investigations and/or treatment to be taken forward for validity assessment (inclusion and exclusion criteria shown in online supplementary figure S1). All full-text scored papers are listed in online supplementary list S1.
annrheumdis-2016-209247supp_figure.pdf (360.3KB, pdf)
annrheumdis-2016-209247supp_list.pdf (306.4KB, pdf)
Validity assessment
A panel of experts (two per paper) independently assessed the methodological quality of papers meeting inclusion criteria (see online supplementary figure S1) and extracted data using predefined scoring forms for diagnostic3 and therapeutic studies.4 Disagreements were resolved by discussion or by the opinion of a third expert. Adapted classification tables for diagnostic,5 therapeutic1 6 and epidemiological studies7 were used to determine the level of evidence and strength of each recommendation.
Establishment of recommendations
As part of the EULAR standard operating procedure, experts described the main results and conclusions of each paper, along with validity and level of evidence. These descriptions were collated by three experts (FBE, LJMC and AvR-K) and used to formulate provisional recommendations (N=65). A summary of the evidence was presented along with each provisional recommendation to the expert committee (n=21) in an online survey (with 100% response rate). Recommendations were revised according to responses and discussed at two sequential face-to-face consensus meetings in March 2014 (Genova, number of experts participating: N=13) and 2015 (Barcelona, number of experts participating: N=15), using Nominal Group Technique.8 A non-voting expert (AR) facilitated the process. Recommendations were accepted when ≥80% of the experts agreed.
Results
Literature review
The literature search yielded 3429 unique papers. After title/abstract and subsequent full-text screening, 115 articles met the inclusion criteria and were selected for quality scoring: 45 articles for therapy, 70 for diagnosis and 3 articles for both groups (detailed in online supplementary figure/list S1). An important manuscript detailing a randomised controlled trial involving treatment with prednisolone, methotrexate (MTX) and ciclosporin was published after the systematic review and consensus meetings, but before submission of this manuscript. With the results of this paper considered, the level of evidence of two recommendations in the therapy section was updated, but the phrasing was not changed.9
Recommendations
The following section describes recommendations with corresponding supporting literature. Tables 1–3 summarise the recommendations, their levels of evidence, recommendation strength and percentage of expert agreement for each. Of note, 39 out of the 59 recommendations accepted are based on expert opinion (level of evidence 4, a strength of evidence D). Recommendations not reaching ≥80% agreement are listed in online supplementary table T1 (N=6).
Table 1.
Overarching principles for juvenile dermatomyositis (JDM)
| L | S | Agreement (%) | |
|---|---|---|---|
| All children with suspected idiopathic inflammatory myopathies should be referred to a specialised centre. | 4 | D | 100 |
High-risk patients need immediate/urgent referral to a specialised centre. High risk patients are defined by
|
4 | D | 100 |
| For JDM, patient-/parent-reported outcome measures are helpful when assessing disease activity and should be used at diagnosis and during disease monitoring. | 4 | D | 100 |
| Validated tools should be used to measure health status, for example, the Childhood Health Assessment Questionnaire, patient/parent visual analogue scale, Childhood Health Questionnaire, Juvenile Dermatomyositis Multi-dimensional Assessment Report. | 4 | D | 82 |
| All children with JDM should have disease activity (muscle, skin, major organ) assessed regularly in a standardised way, using tools such as the Disease Activity Score. | 4 | D | 100 |
| All children with JDM should have disease damage assessed at least yearly using a standardised disease damage measure, such as the Myositis Damage Index. | 4 | D | 100 |
| All patients with JDM should have the opportunity to be registered within a research registry/repository, for example, the Euromyositis registry. | 4 | D | 100 |
Agreement indicates percentage of experts that agreed on the recommendation during the final voting round of the consensus meeting.
1A, meta-analysis of randomised controlled trial; 1B, randomised controlled study; 2A, controlled study without randomisation; 2B, quasi-experimental study; 3, descriptive study; 4 expert opinion; A, based on level 1 evidence; B, based on level 2 or extrapolated from level 1; C, based on level 3 or extrapolated from level 1 or 2; CMAS, Childhood Myositis Assessment Scale; D, based on level 4 or extrapolated from level 3 or 4 expert opinion; L, level of evidence; MMT, Manual Muscle Test; S, strength of recommendation;
Table 2.
Recommendations regarding diagnosis
| L | S | Agreement (%) | |
|---|---|---|---|
| (a) General recommendations | |||
| In the absence of cutaneous signs and/or failure to respond as expected to therapy, alternative diagnoses should be considered including metabolic or mitochondrial myopathies and dystrophies. | 4 | D | 100 |
In every patient in whom a diagnosis of JDM is considered, the following list of investigations should be considered:
|
4 | D | 94 |
| (b) Specific recommendations | |||
| Assessment of muscle involvement | |||
| Both muscle strength and function should be tested at diagnosis and follow up by formal validated measures, such as the MMT8 and the CMAS. | 2a-3 | B–C | 100 |
| MRI can be used to aid diagnosis of JDM. | 2B | B | 100 |
| MRI can be used to help monitor disease activity. | 2B | B | 100 |
| When used, MRI should be carried out by defined protocols that enhance detection of muscle inflammation, such as T2 weighted/STIR sequences. | 3 | C | 100 |
| MRI should be interpreted by an expert radiologist. | 4 | D | 100 |
| A muscle biopsy should be done in all cases where the presentation of JDM is atypical; in particular in the absence of rash/skin signs. | 4 | D | 100 |
| If a muscle biopsy is performed for diagnosis of JDM, a standardised JDM biopsy score tool should be used to quantify severity of histological abnormalities. | 2B | B | 100 |
| Expert histopathological opinion is required to define features of inflammation in JDM muscle biopsy. | 4 | D | 100 |
| When doing a muscle biopsy, there is insufficient evidence to recommend a needle biopsy as opposed to an open biopsy in children. | 3 | C | 100 |
| In cases where MRI or muscle biopsy is not possible, increased muscle echo intensity on muscle ultrasonography (when performed by an experienced sonographer) may be indicative of myositis. | 2B | C | 82 |
| Swallow function should be formally assessed in every patient. The assessment may include a speech and language therapy assessment, video fluoroscopy/barium studies. | 3 | C | 100 |
| EMG or nerve conduction velocity should be considered to differentiate myopathy from neuropathy when diagnosis of JDM is uncertain. | 4 | D | 100 |
| EMG does not detect metabolic myopathies reliably and further workup is required if this diagnosis is suspected. | 3 | D | 100 |
| Assessment of skin involvement | |||
| Assessment of nailfold capillaries should be used to aid diagnosis of JDM. | 2 | B | 100 |
| At time of diagnosis or disease flare, standardised nailfold capillaroscopy assessment is recommended. During follow-up, assessment of nailfold capillaries should be performed regularly. | 3 | C | 100 |
| A formal CAT should be used to aid diagnosis of JDM. | 4 | D | 100 |
| A formal CAT should be used to monitor skin disease activity over time. | 2B | B | 100 |
| Skin tools may include the DAS (skin), MITAX (skin) or CAT. | 4 | D | 100 |
| Assessment of lung involvement | |||
| All patients with JDM should have an assessment of lung involvement at time of diagnosis. | 3 | C | 100 |
| Assessment should include pulmonary function tests, including CO diffusion. If pulmonary function tests are indicative of interstitial lung disease, further investigations (CXR/ HRCT) are needed. | 4 | D | 100 |
| Assessment of cardiac involvement. | |||
| All patients with JDM should have echocardiography and ECG at diagnosis. | 4 | D | 94 |
| Patients at particular risk of cardiac dysfunction should have repeated cardiac evaluation. Risk factors include hypertension, high disease activity 1 year post diagnosis, long-term high corticosteroid burden or chronic ongoing active disease. | 2B | B | 100 |
| Assessment of calcinosis | |||
| Calcinosis should be looked for in all patients with JDM. | 4 | D | 94 |
| Plain radiographs may be used for the evaluation of calcinosis. | 3 | C | 100 |
| Autoantibodies and biomarkers | |||
| We recommend use of muscle enzymes (CPK, LDH, AST) for diagnosis and disease monitoring in JDM, although it must be recognised muscle enzymes may be normal despite active disease. | 4 | D | 100 |
| Measurement of von Willebrand factor does not provide any additional information for diagnosis of JDM. | 3 | C | 100 |
| There is no significant diagnostic benefit gained from measurement of antinuclear antibody in JDM. | 4 | D | 100 |
| Measurement of myositis-specific autoantibodies (such as anti-TIF 1-γ (p155), anti-NXP2/(p140/MJ), anti-MDA5 and anti-SRP) should be considered, when available. | 2A-3 | B–C | 100 |
| In patients with overlap features, measurement of myositis-associated-antibodies such as anti-PmScl, anti-U1-RNP, anti-La (‘SSB’), anti-Ro (‘SSA’) and anti-Sm may be helpful to clarify the diagnosis. | 4 | D | 100 |
| Further validation studies are recommended to define the use of more sensitive biomarkers in JDM. | 4 | D | 100 |
Agreement indicates percentage of experts that agreed on the recommendation during the final voting round of the consensus meeting.
1A, meta-analysis of randomised controlled trial; 1B, randomised controlled study; 2A, controlled study without randomisation; 2B, quasi-experimental study; 3, descriptive study; 4 expert opinion; A, based on level 1 evidence; B, based on level 2 or extrapolated from level 1; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, based on level 3 or extrapolated from level 1 or 2; CAT, Cutaneous Assessment Tool; CMAS, Childhood Myositis Assessment Scale; CO, carbon monoxide; CRP, C-reactive protein; CXR, chest X-ray; D, based on level 4 or extrapolated from level 3 or 4 expert opinion; EMG, electromyogram; ESR, erythrocyte sedimentation rate; HRCT, high-resolution computed tomography; L, level of evidence; LDH, lactate dehydrogenase; MITAX, myositis intention to treat activity index; MMT, Manual Muscle Test; RNP, anti-ribonuclear protein; S, strength of recommendation; SGOT, Serum Glutamic-Oxaloacetic Transaminase; SGPT, Serum Glutamic-Pyruvic Transaminase; SRP, signal recognition particle; SSA, Ro antibodies; SSB, Sjögren's syndrome type B antibodies; STIR, Short-TI Inversion Recovery.
Table 3.
Recommendations regarding treatment
| L | S | Agreement (%) | |
|---|---|---|---|
| Sun protection, including the routine use of sunblock on sun-exposed areas should be encouraged for patients with JDM. | 4 | D | 100 |
| When treating patients with JDM, it is particularly important to have a physiotherapist and a specialist nurse actively involved as part of a multidisciplinary team. | 4 | D | 100 |
| Treatment of JDM should include a safe and appropriate exercise programme, monitored by a physiotherapist. | 4 | D | 100 |
| We recommend the induction regimen for treatment of new onset patients with JDM to be based on high dose of corticosteroids (oral or intravenous) combined with MTX. | 1B | A | 100 |
| High-dose corticosteroids should be administered systemically either orally or intravenously in moderate–severe JDM. | 2A | B | 100 |
| High-dose corticosteroids should be administered intravenously if there are concerns about absorption. | 3 | C | 100 |
| Corticosteroid dose should be weaned as the patient shows clinical improvement. | 4 | D | 100 |
| Addition of MTX or ciclosporin A leads to better disease control than prednisolone alone; safety profiles favour the combination of methotrexate and prednisolone. | 1B | A | 100 |
| MTX should be started at a dose of 15–20 mg/m2/week (max absolute dose of 40 mg /week) preferably administered subcutaneously at disease onset. | 4 | D | 100 |
| If a newly diagnosed patient has inadequate response to treatment, intensification of treatment should be considered within the first 12 weeks, after consultation with an expert centre. | 4 | D | 100 |
| Intravenous immunoglobulin may be a useful adjunct for resistant disease, particularly when skin features are prominent. | 2B-4 | C | 100 |
| MMF may be a useful therapy for muscle and skin disease (including calcinosis). | 3 | C | 100 |
| Ongoing skin disease reflects ongoing systemic disease and therefore should be treated by increasing systemic immunosuppression. Topical tacrolimus (0.1%)/topical steroids may help localised skin disease, particularly for symptomatic redness or itching. | 4 | D | 100 |
| In patients who are intolerant to methotrexate, change to another DMARD, including ciclosporin A or MMF. | 3 | C | 100 |
| For patients with severe disease (such as major organ involvement/extensive ulcerative skin disease), addition of intravenous cyclophosphamide should be considered. | 3 | C | 100 |
| B cell depletion therapy (rituximab) can be considered as an adjunctive therapy for those with refractory disease. Clinicians should be aware that rituximab can take up to 26 weeks to work. | 1B | D | 100 |
| Anti-TNF therapies can be considered in refractory disease; infliximab or adalimumab are favoured over etanercept. | 3 | D | 92 |
| In the presence of developing or established calcinosis, intensification of immunosuppressive therapy should be considered. | 3 | C | 100 |
| There is no high-level evidence of when to stop therapy; however, consideration may be given to withdrawing treatment if a patient has been off steroids and in remission on methotrexate (or alternative DMARD) for a minimum of 1 year. | 4 | D | 100 |
Agreement indicates percentage of experts that agreed on the recommendation during the final voting round of the consensus meeting.
1A, meta-analysis of randomised controlled trial; 1B, randomised controlled study; 2A, controlled study without randomisation; 2B, quasi-experimental study; 3, descriptive study; 4 expert opinion; A, based on level 1 evidence; B, based on level 2 or extrapolated from level 1; C, based on level 3 or extrapolated from level 1 or 2; D, based on level 4 or extrapolated from level 3 or 4 expert opinion; DMARD, disease-modifying antirheumatic drug; JDM, juvenile dermatomyositis; L, level of evidence; MMF, mycophenolate mofetil; MTX, methotrexate; S, strength of recommendation; TNF, tumour necrosis factor.
annrheumdis-2016-209247supp_table.pdf (249.5KB, pdf)
Overarching principles
JDM is the most common idiopathic inflammatory myopathy of childhood, but the incidence is very low; 2–4 cases per million children per year (table 1).10 Standardisation of diagnostic tests and treatment regimens will enable collaborative research studies to increase knowledge of this rare disease.11 JDM vasculopathy principally affects muscles and skin, but may affect other organs and cause constitutional symptoms. With early treatment, 30–50% of patients have the potential to reach remission within 2–3 years of disease onset with few complications and a mortality rate of <4%.12–15 However, polycyclic or persistently active disease has been described in 41–60% of cases in recent cohort studies (depending on activity measures used) and complications like calcinosis, persistent muscle weakness, skin or muscle atrophy remain problematic.12 14–19 Risk of lipodystrophy and calcinosis has been associated with greater duration of active disease and inadequate corticosteroid therapy.15 17 20 21 Quality of life may be impaired compared with healthy controls15 in both physical and psychosocial domains, requiring psychosocial support. In view of the rarity and seriousness of the condition, it was agreed that children with JDM should be cared for in centres with experience and expertise in this condition. Treatment goals include control of disease activity, prevention of organ damage and improvement in quality of life with participation in daily activities. Evaluation of treatment response (including measurement of disease activity or disease damage and monitoring adverse effects of immunosuppressive medication) is an important cornerstone of management.22–24 Many standardised tools, developed primarily for research, are available for this, including the disease activity score (DAS) and myositis disease activity assessment tool.25 It is recognised that registries provide useful resources to investigate rare disease such as JDM.11 In order to better understand prognosis and enhance therapeutic development of this rare disease, the expert group found it important to recommend that all patients JDM should have the opportunity to participate in a research registry.
Recommendations regarding diagnosis
Diagnostic criteria for dermatomyositis, established by Bohan and Peter in 1975, include five items: characteristic skin rash, proximal muscle weakness, raised muscle enzymes, myopathic changes on electromyogram (EMG) and typical muscle biopsy (table 2).26 These are currently being revised through the International Myositis Classification Criteria Project.27 Current practice reveals the necessity of broadening diagnostic criteria by incorporating new techniques, such us MRI and ultrasound, and the significance of skin disease in JDM.14 15 21 28 29 The expert group suggested a non-exhaustive list of investigations for consideration in every patient in whom the diagnosis of JDM is suspected. More specific recommendations have also been established.
Assessment of muscle disease
Muscle strength should be formally tested using validated measures of muscle testing such as the Childhood Myositis Assessment Scale (CMAS) and Manual Muscle Test (MMT). Both tools have been validated as reliable and useful tests to assess muscle strength at diagnosis and follow-up.30–33 They are important outcome measurements in clinical trials and form part of the Paediatric Rheumatology INternational Trials Organization (PRINTO) remission criteria.34 Some of the CMAS manoeuvres are age dependent. It has been shown that healthy children up to 9 years do not always achieve the maximum CMAS score of 52, hence, definition of active disease/remission should include a lower threshold in younger children.35 36
MRI is a reliable tool to assess inflammation in muscle at time of diagnosis and can also help to differentiate active and inactive disease during follow-up.37 Protocols that enhance detection of muscle inflammation, such as T2-weighted (fat-suppressed) imaging techniques,38 should be used. An expert radiologist should evaluate MRI findings.39
Surveys and recent cohort studies demonstrate increasing use of MRI over time (26–89.9%) as a diagnostic modality in contrast to decreasing use of muscle biopsy (36–65%) and EMG (7.6–55.5%)14 15 21 28 29 40 The expert group recommend EMG or nerve conduction velocity only when diagnosis is uncertain. Of note, EMG does not reliably detect metabolic myopathies.41
The expert group advises biopsy when presentation is atypical or diagnosis is in doubt. Use of a standardised JDM biopsy score tool to quantify severity of histological abnormalities is recommended.42 Different markers have been suggested to typify muscle inflammation in patients with JDM, like major histocompatibility complex (MHC) class I, vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), CD59 and toll-like receptor (TLR),43–48 but these need further validation. Expert histopathological opinion is required to define use of these markers for diagnosis of JDM based on muscle biopsy findings. The literature suggests that needle biopsy is a safe and cost-effective alternative to open biopsy in adult patients.49 However, this has not been evaluated sufficiently in children to result in a recommendation.
Ultrasonography has been found in small patients cohorts (7–10 patients) to be a useful tool for myositis.50 51 The expert group suggests that when MRI or muscle biopsy is not possible muscle ultrasonography may be an alternative to assess myositis activity.
The literature suggests that swallowing dysfunction, including silent aspiration, is under-recognised and not always predicted by generalised muscle weakness.52 The expert group recommend that patients at risk of swallowing difficulties (eg, those presenting with nasal speech or coughing during swallowing) should have an objective assessment based on local experience (speech and language therapy assessment, video fluoroscopy/barium studies).
Assessment of skin disease
The expert group recommend use of a cutaneous assessment tool (CAT), including nailfold capillaroscopy to detect periungual capillary changes, as part of assessment of JDM skin activity at diagnosis and follow-up. This can be done in clinic with the aid of magnification using an otoscope, ophthalmoscope or dermatoscope, or defined by formal capillaroscopy. Evidence suggests that nailfold capillary density is a sensitive measure of skin and muscle disease activity.53–58 The importance of residual skin changes in JDM is increasingly recognised, with persistent capillary abnormalities and Gottron's papules at 6 months being associated with longer time to remission.16 The expert group therefore recommend that follow-up of patients with JDM should include use of a CAT. Different tools are available, including the DAS (skin), Myositis Intention to Treat Activity IndeX (MITAX, skin), CAT, Dermatomyositis Skin Severity Index or Cutaneous Dermatomyositis Disease Area and Severity Index; the last two less frequently used in children. There is currently insufficient evidence that any one tool is superior to the other.59–61
Assessment of JDM-associated lung disease
Lung involvement (interstitial lung disease (ILD)) is present in only ∼8% of patients, and often asymptomatic, but assessment is important since ILD is a significant cause of morbidity and mortality.62 The expert group determined that all children should have assessment of lung involvement at time of diagnosis by pulmonary function tests, including carbon monoxide (CO) diffusion capacity.63 64 Further testing is necessary in those with an abnormal restrictive pattern, preferably in collaboration with a paediatric pulmonologist. The performance of pulmonary function tests can be difficult in very young children and can also be affected by general muscle weakness. High-resolution CT is a non-invasive and sensitive test for detecting ILD in JDM, but radiation risk associated with repeated CT scan must be considered.63 There is insufficient evidence to advise on frequency of assessment during follow-up, but physicians should be aware of the long-term risk of lung involvement especially in those with a high myositis damage index (MDI) score62 and a regular assessment of lung function may be prudent in patients that have positive anti-RNA synthetase antibodies.64
Assessment of JDM-associated cardiac disease
Understanding of cardiac manifestations in JDM is limited. One long-term complication is hypertension due to steroid treatment.65 Case studies report the presence of pericarditis, endocarditis and cardiac arrhythmias.66 67 Recent evidence using echocardiography has detected systolic and diastolic dysfunction, particularly in patients with high long-term organ damage scores (MDI, follow-up) and high early skin (but not muscle) disease activity (DAS skin, year 1). Notably, most patients were asymptomatic. The authors of the study suggest that vasculopathy in the myocardium resembles vasculopathy in the skin.65 The long-term clinical consequences of abnormal echocardiographic findings in asymptomatic patients with JDM are unclear. The expert group recommends cardiac evaluation by ECG and echocardiography for all patients. Repeated cardiac evaluation should be considered in patients with high risk of cardiac involvement; risk factors include hypertension, high disease activity 1 year post diagnosis, long-term high corticosteroid burden or chronic ongoing active disease.65 Echocardiac changes are recognised even when patients are in clinical remission65 68 and thus long-term cardiac evaluation should be considered for patients at high risk. There is currently insufficient evidence to advise on frequency and duration of monitoring.
Assessment of calcinosis
Calcinosis is a well-recognised complication in patients with JDM, often occurring later in disease course, on average 2.9 years after disease onset.18 The expert group recommends actively looking for calcinosis by manual palpation, with the use of plain radiographs where needed. CT has been found to have no additional benefit over radiographs for detecting calcinosis.69
Biomarkers and autoantibodies
Muscle enzymes are not always elevated at diagnosis of JDM and are poorly responsive to changing disease activity.70–72 Consensus processes have demonstrated differences in opinion regarding inclusion of muscle enzymes as a core set measure of activity. The International Myositis Association and Clinical Studies Group (IMACS) core set includes muscle enzymes, but the PRINTO core set excludes muscle enzymes due to their poor statistical performance.73 74 Despite these limitations, muscle enzymes are easily accessible and practice surveys suggest that the enzymes are used in routine care at diagnosis and during follow-up.14 15 21 28 29 The expert group recommends measurement of all listed enzymes at diagnosis (table 2) and follow-up as one of them may be elevated in the presence of a normal creatinine phosphokinase (CPK). From current literature, there is no evidence that the von Willebrand factor provides additional information compared with muscle enzymes.75 Several markers of immunological activation appear to correlate with disease activity or potentially with worse disease outcome in JDM, but further studies are needed to determine their sensitivity, like interferon (IFN)-I chemokine signatures76–78 and neopterin.79
Antinuclear antibodies are frequently positive in patients with JDM (prevalence varies through different populations), but no diagnostic value has been established.80 Increasing evidence supports association between serotype and clinical phenotype.81 Myositis-specific autoantibodies target either nuclear or cytoplasmic components involved in gene transcription, protein translocation and antiviral responses. The literature suggests that the presence of anti-p155 (anti-TIF1γ) myositis-associated antibodies (MAA) predicts worse cutaneous involvement, anti-p140 (also known as NXP-2 or MJ), predicts calcinosis, severe disease course and persistent disease activity, and anti-MDA5 is associated with increased risk of skin and oral ulceration, arthritis, milder muscle disease and interstitial lung disease. The association with severe lung disease was most striking in Japanese patients. Anti-signal recognition particle (SRP) is associated with necrotising autoimmune myopathy.20 82–90 The studies mentioned above provide control sera derived from adults and none of these autoantibodies have been validated to date in large patient cohorts. At the time of publication, there is insufficient evidence to recommend measurement of autoantibodies for risk stratification due to lack of validation and data in patients from different ethnicities. However, when available, measurement of myositis-specific antibodies or MAA may be helpful, but should be performed and validated in a laboratory with experience and expertise.
Therapy
Early and aggressive therapy may prevent or stabilise organ damage and disease complications like calcinosis, the latter being associated with significant morbidity due to pain and risk of infection (table 3).14 15 21 71 91–94 JDM treatment is largely based on experience of the treating paediatric rheumatologist. Management is complex and warrants a multidisciplinary approach including physiotherapists, specialist nurses and paediatric rheumatologists, with other specialists, as needed, for example, cardiologist/pulmonologist. The mainstay of therapy is high-dose corticosteroid initially in combination with disease-modifying drugs like MTX or ciclosporin A (CsA).71 95 Evidence for treatment is limited and often confined to small case-controlled studies, with the exception of two randomised controlled trials.9 96 In 2010, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) reached consensus on treatment plans for moderately severe JDM for the first two months after diagnosis that include a combination of steroid (intravenous methylprednisolone followed by oral prednisolone or high-dose oral prednisolone alone) and MTX±intravenous immunoglobulin (IVIG).97
The only randomised controlled trial for newly diagnosed patients was performed by PRINTO from 2006 to 2011, comparing three commonly used protocols (prednisone alone vs combination of prednisone with either MTX or CsA). The combination of steroids and MTX had the best outcome for efficacy and safety.9 In 2013, a randomised controlled trial was published for use of rituximab in refractory myositis, including JDM, in a delayed start design.96 There was no statistically significant difference between treatment groups, but 83% of patients met the definition of improvement by trial completion. These data, with the ability to taper glucocorticoid therapy and good re-treatment response, suggest that rituximab may be useful in refractory cases of myositis.98
The expert group proposed recommendations for treatment of newly diagnosed patients and resistant disease. Treatment of refractory patients with or without calcinosis15 is still a challenge. Treatments used for refractory disease include IVIG, cyclophosphamide, CsA, azathioprine, mycophenolate mofetil (MMF), hydroxychloroquine, tacrolimus, rituximab, infliximab and autologous stem cell transplantation.9 96 98–112 No head-to-head or superiority trial has been carried out.
Published data suggest that early aggressive treatment may decrease incidence of calcinosis.14 15 21 71 91–94 Established calcinosis may respond to treatment with bisphosphonates (pamidronate/alendronate), infliximab, abatacept, diltiazem, probenecid, intravenous immunoglobulins, intralesional steroids or surgical resection.101 111 113–124 Evidence for individual treatments is limited to case reports, except infliximab, diltiazem and pamidronate (case series of five, four and three patients, respectively); therefore, no recommendation regarding a specific treatment of calcinosis was made.
Therapeutic trials are hampered by recruitment of sufficient numbers of patients with JDM, but also by limited tools or biomarkers to measure outcome. Currently, there is no uniform, simple and practical tool to evaluate improvement or inactive disease to guide individual treatment. PRINTO and IMACS have developed preliminary definitions of improvement (DoI) for use in clinical trials, which include multiple assessments of core set measures (CSMs). Although the CSMs used within DoIs differ slightly, PRINTO and IMACS both expect at least 20% improvement in three out of six CSMs with no more than one or two other core set measures getting worse and muscle strength not allowed to worsen.74 125 These definitions and recommendations are best suited to clinical trials, with appropriate infrastructure, but are time consuming in clinical practice. In 2012, CARRA developed a single-consensus steroid taper plan, including when and how to stop steroids.126 However, the SHARE expert group did not agree to a specific steroid-tapering regimen.
In 2012, PRINTO published criteria defining clinically inactive disease; necessitating fulfilment of three out of four variables from CPK ≤150 U/L, CMAS ≥48, MMT8 ≥78 and PGA score of overall disease activity ≤0.2.34 These criteria are weighted towards muscle disease, and when tested in a Norwegian cohort127 CPK was found not to differentiate well between active and inactive disease. When tested in a UK cohort, without PGA, there was a high incidence of skin disease, leading to the suggestion that PGA should be an essential criterion since it is the only measure that includes skin activity.128 The expert group determined that treatment should be escalated if a patient has inadequate response to treatment, including isolated skin disease.
There is no high-level evidence regarding when to stop immunosuppressive therapy. The expert group suggested considering the withdrawal of MTX (or alternative disease-modifying drug) once the patient is in remission and off steroids for a minimum of 1 year.
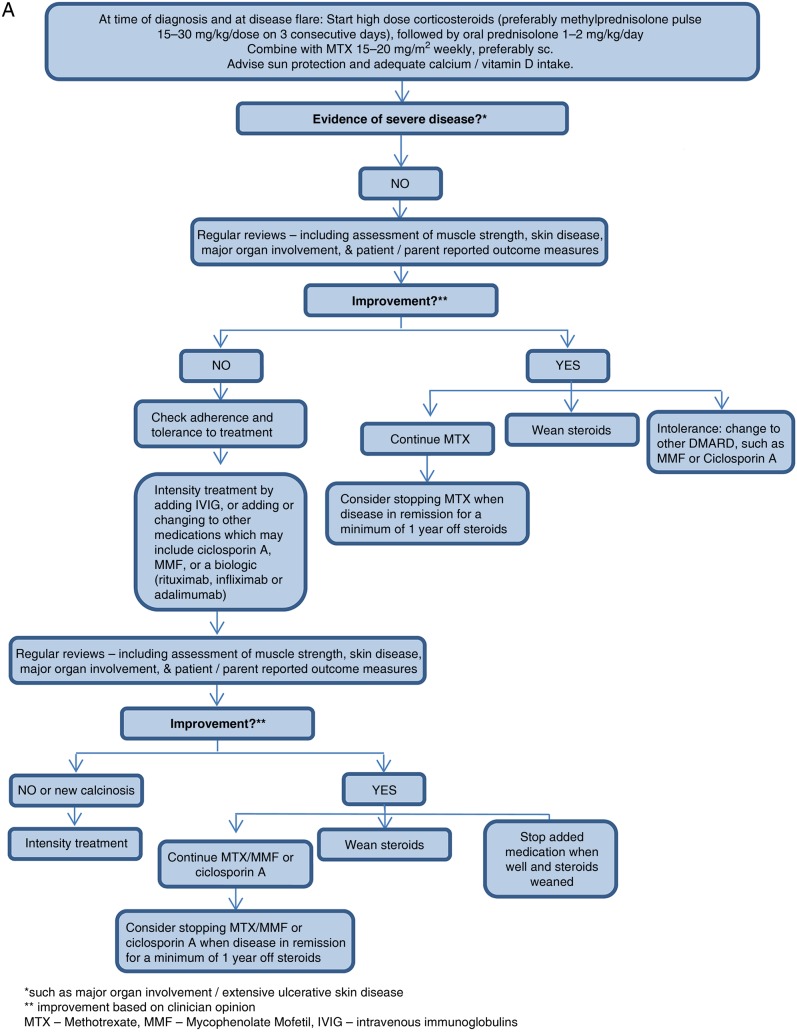
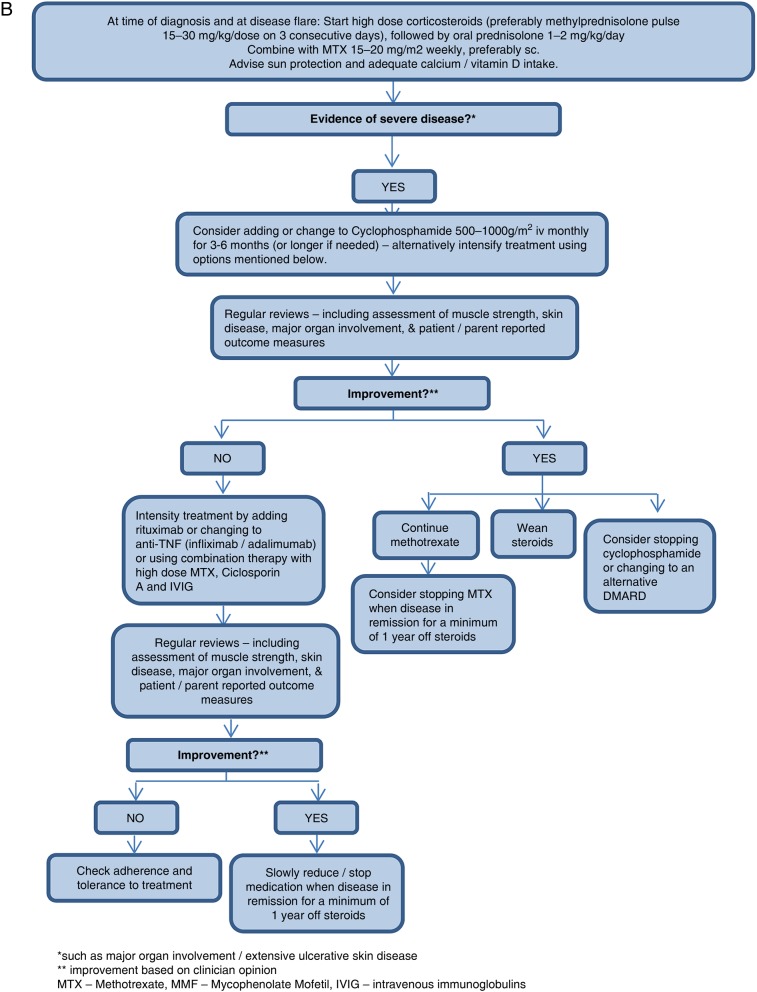
Based on consensus recommendations, a flow chart was established for JDM treatment (figure 1).
Figure 1A.
Flow chart for the treatment of mild/moderate disease in newly diagnosed and refractory patients with juvenile dermatomyositis (JDM).
Figure 1B.
Flow chart for the treatment of severe disease in newly diagnosed and refractory patients with juvenile dermatomyositis (JDM).
Discussion
The JDM working group of SHARE formulated a total of 62 recommendations for the diagnosis and management of JDM, based on a systematic literature review and consensus procedure. In total, 7 overarching principles, 33 recommendations on diagnosis and 19 on therapy were accepted with >80% agreement among the experts. Topics include assessment of skin, muscle and major organ involvement and treatment suggestions at disease onset and in refractory disease.
Diagnostic criteria in JDM are under revision, but will need further adjustment as new outcome tools, especially autoantibodies and biomarkers, are being developed.
Close monitoring of patients' disease status and well-being by an experienced multidisciplinary team is essential for a good clinical outcome. Recent evidence highlights the importance of treating skin disease aggressively as it is associated with high morbidity. Long-term follow-up studies are warranted to clarify complication risks. Given the disease rarity, international collaboration is crucial to recruit sufficient patients. Validated scores for disease activity and damage are needed in order to perform a structured assessment of outcome. Disease activity and damage scores have been developed, principally for clinical trials, but may be challenging and time consuming to use in daily clinical practice.
To conclude, this SHARE initiative is based on expert opinion informed by the best available evidence and provides recommendations for the diagnosis and treatment of patients with JDM, along with other paediatric rheumatic diseases129 with a view to improving the outcome for patients with JDM in Europe. It will now be important to broaden discussion and test acceptability of these to the wider community.
Acknowledgments
This SHARE initiative has been endorsed by the executive committee of the Paediatric Rheumatology European Society and the International Society of Systemic Auto-Inflammatory Diseases.
Footnotes
Twitter: Follow Ricardo Russo at @el_reumatologo
Contributors: LJM and AvR-K are senior authors. NW and SV designed the SHARE initiative. FBE performed the systematic literature review, supervised by LMC and ARK. Validity assessment of selected papers was done by FBE, BB-M, BMF, CP, AR, MvB, JvdN, SV, LRW, NW, LJMC and AvR-K. Recommendations were formulated by FBE, LJMC and AvR-K. The expert committee consisted of FBE, BB-M, EB, TC, PD, BMF, PL, BM, KN, CP, SO, AvR-K, RR, YU, JvdN, NW, LRW, MvB, SV, LJMC and AvR-K; they completed the online surveys and/or participated in the subsequent consensus meetings. AR assisted in the preparation of the two live consensus meetings with FBE, LJMC, AvR-K and facilitated the consensus procedure using nominal group technique. FBE, AvR-K and LJM wrote the manuscript, with contribution and approval of all co-authors.
Funding: This project was supported by a grant from European Agency for Health and Consumers (EAHC), grant number 20111202.
Competing interests: FBE—Valeria e Ettore Bossi Foundation. EB—speaker bureau for Roche/ Chugai, Ad Board for Abbvie and Pfizer. BMF—consultant for Novartis, Pfizer, BMS. PL—consultant for BMS, Pfizer. SO—consultant for Novartis, speaker bureau of SOBI. PD—consultant for Roche, speaker bureau for Pfizer, Novartis, grant support from SOBI, Novartis, Abbvie, Roche, Pfizer, Medac. AR—grant/research support from Pfizer and The Myositis Association; consultant for Novartis, Roche; speaker bureau of Abbvie, Novartis, Pfizer, Roche. YU—consultant for Novartis, speaker bureau of Abbvie, Neopharm, Novartis, Roche. NW—grant/research support from EAHC, Abbvie, GSK, Roche, consultant for Genzyme, Novartis, Pfizer, Roche
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dougados M, Betteridge N, Burmester GR, et al. . EULAR standardised operating procedures for the elaboration, evaluation, dissemination, and implementation of recommendations endorsed by the EULAR standing committees. Ann Rheum Dis 2004;63:1172–6. 10.1136/ard.2004.023697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijde D, Aletaha D, Carmona L, et al. . 2014 Update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis 2015;74:8–13. 10.1136/annrheumdis-2014-206350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiting P, Rutjes AW, Dinnes J, et al. . Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 2004;8:iii, 1–234 10.3310/hta8250 [DOI] [PubMed] [Google Scholar]
- 4.Collaboration, T. C. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2013.
- 5.Zhang W, Doherty M, Pascual E, et al. . EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006;65:1301–11. 10.1136/ard.2006.055251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Doherty M, Bardin T, et al. . EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006;65:1312–24. 10.1136/ard.2006.055269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Group OCEBMLOEW. The Oxford Levels of Evidence 1. 2007. http://www.cebm.net/ocebm-levels-of-evidence/
- 8.Van de Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health 1972;62:337–42. 10.2105/AJPH.62.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruperto N, Pistorio A, Oliveira S, et al. . Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet 2016;387:671–8. 10.1016/S0140-6736(15)01021-1 [DOI] [PubMed] [Google Scholar]
- 10.Meyer A, Meyer N, Schaeffer M, et al. . Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology (Oxford) 2015;54:50–63. 10.1093/rheumatology/keu289 [DOI] [PubMed] [Google Scholar]
- 11.Lundberg IE, Svensson J. Registries in idiopathic inflammatory myopathies. Curr Opin Rheumatol 2013;25:729–34. 10.1097/01.bor.0000434667.55020.e1 [DOI] [PubMed] [Google Scholar]
- 12.Huber AM, Lang B, LeBlanc CM, et al. . Medium- and long-term functional outcomes in a multicenter cohort of children with juvenile dermatomyositis. Arthritis Rheum 2000;43:541–9. [DOI] [PubMed] [Google Scholar]
- 13.Gowdie PJ, Allen RC, Kornberg AJ, et al. . Clinical features and disease course of patients with juvenile dermatomyositis. Int J Rheum Dis 2013;16:561–7. 10.1111/1756-185X.12107 [DOI] [PubMed] [Google Scholar]
- 14.Mathiesen PR, Zak M, Herlin T, et al. . Clinical features and outcome in a Danish cohort of juvenile dermatomyositis patients. Clin Exp Rheumatol 2010;28:782–9. [PubMed] [Google Scholar]
- 15.Ravelli A, Trail L, Ferrari C, et al. . Long-term outcome and prognostic factors of juvenile dermatomyositis: a multinational, multicenter study of 490 patients. Arthritis Care Res (Hoboken) 2010;62:63–72. 10.1002/acr.20015 [DOI] [PubMed] [Google Scholar]
- 16.Stringer E, Singh-Grewal D, Feldman BM. Predicting the course of juvenile dermatomyositis: significance of early clinical and laboratory features. Arthritis Rheum 2008;58:3585–92. 10.1002/art.23960 [DOI] [PubMed] [Google Scholar]
- 17.Sanner H, Gran JT, Sjaastad I, et al. . Cumulative organ damage and prognostic factors in juvenile dermatomyositis: a cross-sectional study median 16.8 years after symptom onset. Rheumatology (Oxford) 2009;48:1541–7. 10.1093/rheumatology/kep302 [DOI] [PubMed] [Google Scholar]
- 18.Balin SJ, Wetter DA, Andersen LK, et al. . Calcinosis cutis occurring in association with autoimmune connective tissue disease: the Mayo Clinic experience with 78 patients, 1996–2009. Arch Dermatol 2012;148:455–62. 10.1001/archdermatol.2011.2052 [DOI] [PubMed] [Google Scholar]
- 19.Robinson AB, Reed AM. Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nat Rev Rheumatol 2011;7:664–75. 10.1038/nrrheum.2011.139 [DOI] [PubMed] [Google Scholar]
- 20.Rider LG, Shah M, Mamyrova G, et al. . The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92:223–43. 10.1097/MD.0b013e31829d08f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson AB, Hoeltzel MF, Wahezi DM, et al. . Clinical characteristics of children with juvenile dermatomyositis: the Childhood Arthritis and Rheumatology Research Alliance Registry. Arthritis Care Res (Hoboken) 2014;66:404–10. 10.1002/acr.22142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luca N, Feldman BM. Pediatric rheumatology: Improving the assessment of children with JIA. Nat Rev Rheumatol 2011;7:442–4. 10.1038/nrrheum.2011.99 [DOI] [PubMed] [Google Scholar]
- 23.Luca NJ, Feldman BM. Disease activity measures in paediatric rheumatic diseases. Int J Rheumatol 2013;2013:715352 10.1155/2013/715352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luca NJ, Feldman BM. Health outcomes of pediatric rheumatic diseases. Best Pract Res Clin Rheumatol 2014;28:331–50. 10.1016/j.berh.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 25.Rider LG, Werth VP, Huber AM, et al. . Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S118–57. 10.1002/acr.20532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. 10.1056/NEJM197502132920706 [DOI] [PubMed] [Google Scholar]
- 27.Amato AA, Barohn R, Devisser M, et al. . The International myositis classification criteria. 2005. https://www.niehs.nih.gov/research/resources/assets/docs/2005_working_committee_meeting_summary_508.pdf
- 28.Brown VE, Pilkington CA, Feldman BM, et al. . An international consensus survey of the diagnostic criteria for juvenile dermatomyositis (JDM). Rheumatology (Oxford) 2006;45:990–3. 10.1093/rheumatology/kel025 [DOI] [PubMed] [Google Scholar]
- 29.Martin N, Krol P, Smith S, et al. . A national registry for juvenile dermatomyositis and other paediatric idiopathic inflammatory myopathies: 10 years’ experience; the Juvenile Dermatomyositis National (UK and Ireland) Cohort Biomarker Study and Repository for Idiopathic Inflammatory Myopathies. Rheumatology (Oxford) 2011;50:137–45. 10.1093/rheumatology/keq261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber AM, Feldman BM, Rennebohm RM, et al. . Validation and clinical significance of the Childhood Myositis Assessment Scale for assessment of muscle function in the juvenile idiopathic inflammatory myopathies. Arthritis Rheum 2004;50: 1595–603. 10.1002/art.20179 [DOI] [PubMed] [Google Scholar]
- 31.Lovell DJ, Lindsley CB, Rennebohm RM, et al. . Development of validated disease activity and damage indices for the juvenile idiopathic inflammatory myopathies. II. The Childhood Myositis Assessment Scale (CMAS): a quantitative tool for the evaluation of muscle function. The Juvenile Dermatomyositis Disease Activity Collaborative Study Group. Arthritis Rheum 1999;42:2213–19. [DOI] [PubMed] [Google Scholar]
- 32.Harris-Love MO, Shrader JA, Koziol D, et al. . Distribution and severity of weakness among patients with polymyositis, dermatomyositis and juvenile dermatomyositis. Rheumatology (Oxford) 2009;48:134–9. 10.1093/rheumatology/ken441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain M, Smith M, Cintas H, et al. . Intra-rater and inter-rater reliability of the 10-point Manual Muscle Test (MMT) of strength in children with juvenile idiopathic inflammatory myopathies (JIIM). Phys Occup Ther Pediatr 2006;26:5–17. 10.1080/J006v26n03_02 [DOI] [PubMed] [Google Scholar]
- 34.Lazarevic D, Pistorio A, Palmisani E, et al. . The PRINTO criteria for clinically inactive disease in juvenile dermatomyositis. Ann Rheum Dis 2013;72:686–93. 10.1136/annrheumdis-2012-201483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quiñones R, Morgan GA, Amoruso M, et al. . Lack of achievement of a full score on the childhood myositis assessment scale by healthy four-year-olds and those recovering from juvenile dermatomyositis. Arthritis Care Res (Hoboken) 2013;65:1697–701. 10.1002/acr.22041 [DOI] [PubMed] [Google Scholar]
- 36.Rennebohm RM, Jones K, Huber AM, et al. . Normal scores for nine maneuvers of the Childhood Myositis Assessment Scale. Arthritis Rheum 2004;51:365–70. 10.1002/art.20397 [DOI] [PubMed] [Google Scholar]
- 37.Malattia C, Damasio MB, Madeo A, et al. . Whole-body MRI in the assessment of disease activity in juvenile dermatomyositis. Ann Rheum Dis 2014;73: 1083–90. 10.1136/annrheumdis-2012-202915 [DOI] [PubMed] [Google Scholar]
- 38.Hernandez RJ, Sullivan DB, Chenevert TL, et al. . MR imaging in children with dermatomyositis: musculoskeletal findings and correlation with clinical and laboratory findings. AJR Am J Roentgenol 1993;161:359–66. 10.2214/ajr.161.2.8333378 [DOI] [PubMed] [Google Scholar]
- 39.Davis WR, Halls JE, Offiah AC, et al. . Assessment of active inflammation in juvenile dermatomyositis: a novel magnetic resonance imaging-based scoring system. Rheumatology (Oxford) 2011;50:2237–44. 10.1093/rheumatology/ker262 [DOI] [PubMed] [Google Scholar]
- 40.McCann LJ, Juggins AD, Maillard SM, et al. . The Juvenile Dermatomyositis National Registry and Repository (UK and Ireland)--clinical characteristics of children recruited within the first 5 yr. Rheumatology (Oxford) 2006;45:1255–60. 10.1093/rheumatology/kel099 [DOI] [PubMed] [Google Scholar]
- 41.Ghosh PS, Sorenson EJ. Diagnostic yield of electromyography in children with myopathic disorders. Pediatr Neurol 2014;51:215–19. 10.1016/j.pediatrneurol.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 42.Wedderburn LR, Varsani H, Li CK, et al. . International consensus on a proposed score system for muscle biopsy evaluation in patients with juvenile dermatomyositis: a tool for potential use in clinical trials. Arthritis Rheum 2007;57:1192–201. 10.1002/art.23012 [DOI] [PubMed] [Google Scholar]
- 43.Cappelletti C, Baggi F, Zolezzi F, et al. . Type I interferon and Toll-like receptor expression characterizes inflammatory myopathies. Neurology 2011;76:2079–88. 10.1212/WNL.0b013e31821f440a [DOI] [PubMed] [Google Scholar]
- 44.Sallum AM, Kiss MH, Silva CA, et al. . MHC class I and II expression in juvenile dermatomyositis skeletal muscle. Clin Exp Rheumatol 2009;27:519–26. [PubMed] [Google Scholar]
- 45.Shinjo SK, Sallum AM, Silva CA, et al. . Skeletal muscle major histocompatibility complex class I and II expression differences in adult and juvenile dermatomyositis. Clinics (Sao Paulo) 2012;67:885–90. 10.6061/clinics/2012(08)05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goncalves FG, Chimelli L, Sallum AM, et al. . Immunohistological analysis of CD59 and membrane attack complex of complement in muscle in juvenile dermatomyositis. J Rheumatol 2002;29:1301–7. [PubMed] [Google Scholar]
- 47.Li CK, Varsani H, Holton JL, et al. . MHC Class I overexpression on muscles in early juvenile dermatomyositis. J Rheumatol 2004;31:605–9. [PubMed] [Google Scholar]
- 48.Sallum AM, Kiss MH, Silva CA, et al. . Difference in adhesion molecule expression (ICAM-1 and VCAM-1) in juvenile and adult dermatomyositis, polymyositis and inclusion body myositis. Autoimmun Rev 2006;5:93–100. 10.1016/j.autrev.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 49.Newman ED, Garbes AD. Needle muscle biopsy. J Clin Rheumatol 1997;3:69–74. 10.1097/00124743-199704000-00002 [DOI] [PubMed] [Google Scholar]
- 50.Bhansing KJ, Hoppenreijs EP, Janssen AJ, et al. . Quantitative muscle ultrasound: a potential tool for assessment of disease activity in juvenile ermatomyositis. Scand J Rheumatol 2014;43:339–41. 10.3109/03009742.2013.879674 [DOI] [PubMed] [Google Scholar]
- 51.Habers GE, Van Brussel M, Bhansing KJ, et al. . Quantitative muscle ultrasonography in the follow-up of juvenile dermatomyositis. Muscle Nerve 2015;52:540–6. 10.1002/mus.24564 [DOI] [PubMed] [Google Scholar]
- 52.McCann LJ, Garay SM, Ryan MM, et al. . Oropharyngeal dysphagia in juvenile dermatomyositis (JDM): an evaluation of videofluoroscopy swallow study (VFSS) changes in relation to clinical symptoms and objective muscle scores. Rheumatology (Oxford) 2007;46:1363–6. 10.1093/rheumatology/kem131 [DOI] [PubMed] [Google Scholar]
- 53.Dolezalova P, Young SP, Bacon PA, et al. . Nailfold capillary microscopy in healthy children and in childhood rheumatic diseases: a prospective single blind observational study. Ann Rheum Dis 2003;62:444–9. 10.1136/ard.62.5.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ingegnoli F, Zeni S, Gerloni V, et al. . Capillaroscopic observations in childhood rheumatic diseases and healthy controls. Clin Exp Rheumatol 2005;23:905–11. [PubMed] [Google Scholar]
- 55.Nascif AK, Terreri MT, Len CA, et al. . Inflammatory myopathies in childhood: correlation between nailfold capillaroscopy findings and clinical and laboratory data. J Pediatr (Rio J) 2006;82:40–5. 10.2223/JPED.1435 [DOI] [PubMed] [Google Scholar]
- 56.Piotto DG, Len CA, Hilario MO, et al. . Nailfold capillaroscopy in children and adolescents with rheumatic diseases. Rev Bras Reumatol 2012;52:722–32. 10.1590/S0482-50042012000500007 [DOI] [PubMed] [Google Scholar]
- 57.Scheja A, Elborgh R, Wildt M. Decreased capillary density in juvenile dermatomyositis and in mixed connective tissue disease. J Rheumatol 1999;26:1377–81. [PubMed] [Google Scholar]
- 58.Schmeling H, Stephens S, Goia C, et al. . Nailfold capillary density is importantly associated over time with muscle and skin disease activity in juvenile dermatomyositis. Rheumatology (Oxford) 2011;50:885–93. 10.1093/rheumatology/keq407 [DOI] [PubMed] [Google Scholar]
- 59.Huber AM, Dugan EM, Lachenbruch PA, et al. . The Cutaneous Assessment Tool: development and reliability in juvenile idiopathic inflammatory myopathy. Rheumatology (Oxford) 2007;46:1606–11. 10.1093/rheumatology/kem179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber AM, Lachenbruch PA, Dugan EM, et al. . Alternative scoring of the Cutaneous Assessment Tool in juvenile dermatomyositis: results using abbreviated formats. Arthritis Rheum 2008;59:352–6. 10.1002/art.23313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber AM, Dugan EM, Lachenbruch PA, et al. . Preliminary validation and clinical meaning of the Cutaneous Assessment Tool in juvenile dermatomyositis. Arthritis Rheum 2008;59:214–21. 10.1002/art.23340 [DOI] [PubMed] [Google Scholar]
- 62.Mathiesen PR, Buchvald F, Nielsen KG, et al. . Pulmonary function and autoantibodies in a long-term follow-up of juvenile dermatomyositis patients. Rheumatology (Oxford) 2014;53:644–9. 10.1093/rheumatology/ket380 [DOI] [PubMed] [Google Scholar]
- 63.Pouessel G, Deschildre A, Le Bourgeois M, et al. . The lung is involved in juvenile dermatomyositis. Pediatr Pulmonol 2013;48:1016–25. 10.1002/ppul.22742 [DOI] [PubMed] [Google Scholar]
- 64.Prestridge A, Morgan G, Ferguson L, et al. . Pulmonary function tests in idiopathic inflammatory myopathy: association with clinical parameters in children. Arthritis Care Res (Hoboken) 2013;65:1424–31. 10.1002/acr.22014 [DOI] [PubMed] [Google Scholar]
- 65.Schwartz T, Sanner H, Gjesdal O, et al. . In juvenile dermatomyositis, cardiac systolic dysfunction is present after long-term follow-up and is predicted by sustained early skin activity. Ann Rheum Dis 2014;73:1805–10. [DOI] [PubMed] [Google Scholar]
- 66.Karaca NE, Aksu G, Yeniay BS, et al. . Juvenile dermatomyositis with a rare and remarkable complication: sinus bradycardia. Rheumatol Int 2006;27:179–82. 10.1007/s00296-006-0191-7 [DOI] [PubMed] [Google Scholar]
- 67.Pereira RM, Lerner S, Maeda WT, et al. . Pericardial tamponade in juvenile dermatomyositis. Clin Cardiol 1992;15:301–3. 10.1002/clc.4960150415 [DOI] [PubMed] [Google Scholar]
- 68.Schwartz T, Sanner H, Husebye T, et al. . Cardiac dysfunction in juvenile dermatomyositis: a case-control study. Ann Rheum Dis 2011;70:766–71. 10.1136/ard.2010.137968 [DOI] [PubMed] [Google Scholar]
- 69.Shahi V, Wetter DA, Howe BM, et al. . Plain radiography is effective for the detection of calcinosis cutis occurring in association with autoimmune connective tissue disease. Br J Dermatol 2014;170:1073–9. 10.1111/bjd.12785 [DOI] [PubMed] [Google Scholar]
- 70.Guzman J, Petty RE, Malleson PN. Monitoring disease activity in juvenile dermatomyositis: the role of von Willebrand factor and muscle enzymes. J Rheumatol 1994;21:739–43. [PubMed] [Google Scholar]
- 71.Ramanan AV, Campbell-Webster N, Ota S, et al. . The effectiveness of treating juvenile dermatomyositis with methotrexate and aggressively tapered corticosteroids. Arthritis Rheum 2005;52:3570–8. 10.1002/art.21378 [DOI] [PubMed] [Google Scholar]
- 72.Rider LG. Assessment of disease activity and its sequelae in children and adults with myositis. Curr Opin Rheumatol 1996;8:495–506. 10.1097/00002281-199611000-00002 [DOI] [PubMed] [Google Scholar]
- 73.Ruperto N, Ravelli A, Pistorio A, et al. . The provisional Paediatric Rheumatology International Trials Organisation/American College of Rheumatology/European League Against Rheumatism Disease activity core set for the evaluation of response to therapy in juvenile dermatomyositis: a prospective validation study. Arthritis Rheum 2008;59:4–13. 10.1002/art.23248 [DOI] [PubMed] [Google Scholar]
- 74.Ruperto N, Pistorio A, Ravelli A, et al. . The Paediatric Rheumatology International Trials Organisation provisional criteria for the evaluation of response to therapy in juvenile dermatomyositis. Arthritis Care Res (Hoboken) 2010;62:1533–41. 10.1002/acr.20280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bloom BJ, Tucker LB, Miller LC, et al. . von Willebrand factor in juvenile dermatomyositis. J Rheumatol 1995;22:320–5. [PubMed] [Google Scholar]
- 76.Bilgic H, Ytterberg SR, Amin S, et al. . Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum 2009;60:3436–46. 10.1002/art.24936 [DOI] [PubMed] [Google Scholar]
- 77.Reed AM, Peterson E, Bilgic H, et al. . Changes in novel biomarkers of disease activity in juvenile and adult dermatomyositis are sensitive biomarkers of disease course. Arthritis Rheum 2012;64:4078–86. 10.1002/art.34659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bellutti Enders F, van Wijk F, Scholman R, et al. . Correlation of CXCL10, tumor necrosis factor receptor type II, and galectin 9 with disease activity in juvenile dermatomyositis. Arthritis Rheumatol 2014;66:2281–9. 10.1002/art.38676 [DOI] [PubMed] [Google Scholar]
- 79.Rider LG, Schiffenbauer AS, Zito M, et al. . Neopterin and quinolinic acid are surrogate measures of disease activity in the juvenile idiopathic inflammatory myopathies. Clin Chem 2002;48:1681–8. [PubMed] [Google Scholar]
- 80.Pachman LM, Friedman JM, Maryjowski-Sweeney ML, et al. . Immunogenetic studies of juvenile dermatomyositis. III. Study of antibody to organ-specific and nuclear antigens. Arthritis Rheum 1985;28:151–7. 10.1002/art.1780280208 [DOI] [PubMed] [Google Scholar]
- 81.Gunawardena H. The clinical features of myositis-associated autoantibodies: a review. Clin Rev Allergy Immunol. 10.1007/s12016-015-8513-8 [DOI] [PubMed] [Google Scholar]
- 82.Tansley SL, McHugh NJ. Myositis specific and associated autoantibodies in the diagnosis and management of juvenile and adult idiopathic inflammatory myopathies. Curr Rheumatol Rep 2014;16:464 10.1007/s11926-014-0464-1 [DOI] [PubMed] [Google Scholar]
- 83.Ishikawa A, Muro Y, Sugiura K, et al. . Development of an ELISA for detection of autoantibodies to nuclear matrix protein 2. Rheumatology (Oxford) 2012;51:1181–7. 10.1093/rheumatology/kes033 [DOI] [PubMed] [Google Scholar]
- 84.Tansley SL, Betteridge ZE, Shaddick G, et al. . Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology (Oxford) 2014;53:2204–8. 10.1093/rheumatology/keu259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bodoki L, Nagy-Vincze M, Griger Z, et al. . Four dermatomyositis-specific autoantibodies-anti-TIF1γ, anti-NXP2, anti-SAE and anti-MDA5-in adult and juvenile patients with idiopathic inflammatory myopathies in a Hungarian cohort. Autoimmun Rev 2014;13:1211–19. 10.1016/j.autrev.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 86.Espada G, Maldonado Cocco JA, Fertig N, et al. . Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J Rheumatol. 2009;36:2547–51. 10.3899/jrheum.090461 [DOI] [PubMed] [Google Scholar]
- 87.Gunawardena H, Wedderburn LR, North J, et al. . Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology (Oxford) 2008;47:324–8. 10.1093/rheumatology/kem359 [DOI] [PubMed] [Google Scholar]
- 88.Gunawardena H, Wedderburn LR, Chinoy H, et al. . Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum 2009;60:1807–14. 10.1002/art.24547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kobayashi I, Okura Y, Yamada M, et al. . Anti-melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J Pediatr 2011;158:675–7. 10.1016/j.jpeds.2010.11.033 [DOI] [PubMed] [Google Scholar]
- 90.Tansley SL, Betteridge ZE, Gunawardena H, et al. . Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther 2014;16:R138 10.1186/ar4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fisler RE, Liang MG, Fuhlbrigge RC, et al. . Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol 2002;47:505–11. 10.1067/mjd.2002.122196 [DOI] [PubMed] [Google Scholar]
- 92.Al-Mayouf S, Al-Mazyed A, Bahabri S. Efficacy of early treatment of severe juvenile dermatomyositis with intravenous methylprednisolone and methotrexate. Clin Rheumatol 2000;19:138–41. 10.1007/s100670050032 [DOI] [PubMed] [Google Scholar]
- 93.Miller LC, Sisson BA, Tucker LB, et al. . Methotrexate treatment of recalcitrant childhood dermatomyositis. Arthritis Rheum 1992;35:1143–9. 10.1002/art.1780351006 [DOI] [PubMed] [Google Scholar]
- 94.Fischer TJ, Rachelefsky GS, Klein RB, et al. . Childhood dermatomyositis and polymyositis. Treatment with methotrexate and prednisone. Am J Dis Child 1979;133:386–9. 10.1001/archpedi.1979.02130040040009 [DOI] [PubMed] [Google Scholar]
- 95.Hasija R, Pistorio A, Ravelli A, et al. . Therapeutic approaches in the treatment of juvenile dermatomyositis in patients with recent-onset disease and in those experiencing disease flare: an international multicenter PRINTO study. Arthritis Rheum 2011;63:3142–52. 10.1002/art.30475 [DOI] [PubMed] [Google Scholar]
- 96.Oddis CV, Reed AM, Aggarwal R, et al. . Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 2013;65: 314–24. 10.1002/art.37754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huber AM, Giannini EH, Bowyer SL, et al. . Protocols for the initial treatment of moderately severe juvenile dermatomyositis: results of a Children's Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res (Hoboken) 2010;62:219–25. 10.1002/acr.20071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aggarwal R, Bandos A, Reed AM, et al. . Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol 2014;66:740–9. 10.1002/art.38270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al-Mayouf SM, Laxer RM, Schneider R, et al. . Intravenous immunoglobulin therapy for juvenile dermatomyositis: efficacy and safety. J Rheumatol 2000;27:2498–503. [PubMed] [Google Scholar]
- 100.Lam CG, Manlhiot C, Pullenayegum EM, et al. . Efficacy of intravenous Ig therapy in juvenile dermatomyositis. Ann Rheum Dis 2011;70:2089–94. 10.1136/ard.2011.153718 [DOI] [PubMed] [Google Scholar]
- 101.Yang M-C, Lee J-H, Yang Y-H, et al. . Improvement of juvenile dermatomyositis with calcinosis universalis after treatment with intravenous immunoglobulin. Int J Rheum Dis 2008;11:77–80. 10.1111/j.1756-185X.2008.00336.x [DOI] [Google Scholar]
- 102.Riley P, Maillard SM, Wedderburn LR, et al. . Intravenous cyclophosphamide pulse therapy in juvenile dermatomyositis. A review of efficacy and safety. Rheumatology (Oxford) 2004;43:491–6. 10.1093/rheumatology/keh082 [DOI] [PubMed] [Google Scholar]
- 103.Heckmatt J, Hasson N, Saunders C, et al. . Cyclosporin in juvenile dermatomyositis. Lancet 1989;1:1063–6. 10.1016/S0140-6736(89)92456-2 [DOI] [PubMed] [Google Scholar]
- 104.Jacobs JC. Methotrexate and azathioprine treatment of childhood dermatomyositis. Pediatrics 1977;59:212–8. [PubMed] [Google Scholar]
- 105.Miller LC, Michael AF, Kim Y. Childhood dermatomyositis. Clinical course and long-term follow-up. Clin Pediatr (Phila) 1987;26:561–6. 10.1177/000992288702601101 [DOI] [PubMed] [Google Scholar]
- 106.Ng YT, Ouvrier RA, Wu T. Drug therapy in juvenile dermatomyositis: follow-up study. J Child Neurol 1998;13:109–12. 10.1177/088307389801300303 [DOI] [PubMed] [Google Scholar]
- 107.Rouster-Stevens KA, Morgan GA, Wang D, et al. . Mycophenolate mofetil: a possible therapeutic agent for children with juvenile dermatomyositis. Arthritis Care Res (Hoboken) 2010;62:1446–51. 10.1002/acr.20269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olson NY, Lindsley CB. Adjunctive use of hydroxychloroquine in childhood dermatomyositis. J Rheumatol 1989;16:1545–7. [PubMed] [Google Scholar]
- 109.Hassan J, van der Net JJ, van Royen-Kerkhof A. Treatment of refractory juvenile dermatomyositis with tacrolimus. Clin Rheumatol 2008;27:1469–71. 10.1007/s10067-008-0973-2 [DOI] [PubMed] [Google Scholar]
- 110.Bader-Meunier B, Decaluwe H, Barnerias C, et al. . Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol 2011;38: 1436–40. 10.3899/jrheum.101321 [DOI] [PubMed] [Google Scholar]
- 111.Riley P, McCann LJ, Maillard SM, et al. . Effectiveness of infliximab in the treatment of refractory juvenile dermatomyositis with calcinosis. Rheumatology (Oxford) 2008;47:877–80. 10.1093/rheumatology/ken074 [DOI] [PubMed] [Google Scholar]
- 112.Holzer U, van Royen-Kerkhof A, van der Torre P, et al. . Successful autologous stem cell transplantation in two patients with juvenile dermatomyositis. Scand J Rheumatol 2010;39:88–92. 10.3109/03009740903096622 [DOI] [PubMed] [Google Scholar]
- 113.Ambler GR, Chaitow J, Rogers M, et al. . Rapid improvement of calcinosis in juvenile dermatomyositis with alendronate therapy. J Rheumatol 2005;32: 1837–9. [PubMed] [Google Scholar]
- 114.Arabshahi B, Silverman RA, Jones OY, et al. . Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J Pediatr 2012;160:520–2. 10.1016/j.jpeds.2011.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bertorini TE, Sebes JI, Palmieri GM, et al. . Diltiazem in the treatment of calcinosis in juvenile dermatomyositis. J Clin Neuromuscul Dis 2001;2:191–3. 10.1097/00131402-200106000-00005 [DOI] [PubMed] [Google Scholar]
- 116.Harel L, Harel G, Korenreich L, et al. . Treatment of calcinosis in juvenile dermatomyositis with probenecid: the role of phosphorus metabolism in the development of calcifications. J Rheumatol 2001;28:1129–32. [PubMed] [Google Scholar]
- 117.Ichiki Y, Akiyama T, Shimozawa N, et al. . An extremely severe case of cutaneous calcinosis with juvenile dermatomyositis, and successful treatment with diltiazem. Br J Dermatol 2001;144:894–7. 10.1046/j.1365-2133.2001.04153.x [DOI] [PubMed] [Google Scholar]
- 118.Jazayeri SB, Mehdizadeh M, Shahlaee A. Sketched x-rays: Calcinosis universalis. Eur J Pediatr 2012;171:1577–8. 10.1007/s00431-012-1801-x [DOI] [PubMed] [Google Scholar]
- 119.Jiang X, Yi Q, Liu D, et al. . A case of juvenile dermatomyositis with severe calcinosis and successful treatment with prednisone and diltiazem. Int J Dermatol 2011;50:74–7. 10.1111/j.1365-4632.2009.04449.x [DOI] [PubMed] [Google Scholar]
- 120.Kaliyadan F, Venkitakrishnan S. Late onset calcification following juvenile dermatomyositis: response with weekly alendronate. Indian J Dermatol 2011;56:357–9. 10.4103/0019-5154.82491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marco Puche A, Calvo Penades I, Lopez Montesinos B. Effectiveness of the treatment with intravenous pamidronate in calcinosis in juvenile dermatomyositis. Clin Exp Rheumatol 2010;28:135–40. [PubMed] [Google Scholar]
- 122.Nakamura H, Kawakami A, Ida H, et al. . Efficacy of probenecid for a patient with juvenile dermatomyositis complicated with calcinosis. J Rheumatol 2006;33:1691–3. [PubMed] [Google Scholar]
- 123.Slimani S, Abdessemed A, Haddouche A, et al. . Complete resolution of universal calcinosis in a patient with juvenile dermatomyositis using pamidronate. Joint Bone Spine 2010;77:70–2. 10.1016/j.jbspin.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 124.Touimy M, Janani S, Rachidi W, et al. . Calcinosis universalis complicating juvenile dermatomyositis: improvement after intravenous immunoglobulin therapy. Joint Bone Spine 2013;80:108–9. 10.1016/j.jbspin.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 125.Rider LG, Giannini EH, Brunner HI, et al. . International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum 2004;50:2281–90. 10.1002/art.20349 [DOI] [PubMed] [Google Scholar]
- 126.Huber AM, Robinson AB, Reed AM, et al. . Consensus treatments for moderate juvenile dermatomyositis: beyond the first two months. Results of the second Childhood Arthritis and Rheumatology Research Alliance consensus conference. Arthritis Care Res (Hoboken) 2012;64:546–53. 10.1002/acr.20695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sanner H, Sjaastad I, Flatø B. Disease activity and prognostic factors in juvenile dermatomyositis: a long-term follow-up study applying the Paediatric Rheumatology International Trials Organization criteria for inactive disease and the myositis disease activity assessment tool. Rheumatology (Oxford) 2014;53:1578–85. 10.1093/rheumatology/keu146 [DOI] [PubMed] [Google Scholar]
- 128.Almeida B, Campanilho-Marques R, Arnold K, et al. . Analysis of published criteria for clinically inactive disease in a large juvenile dermatomyositis cohort shows that skin disease is underestimated. Arthritis Rheumatol 2015;67:2495–502. 10.1002/art.39200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wulffraat NM, Vastert B, SHARE C. Time to share. Pediatr Rheumatol Online J 2013;11:5 10.1186/1546-0096-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2016-209247supp_figure.pdf (360.3KB, pdf)
annrheumdis-2016-209247supp_list.pdf (306.4KB, pdf)
annrheumdis-2016-209247supp_table.pdf (249.5KB, pdf)