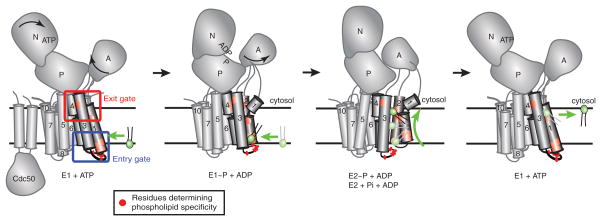
Figure 2. P4-ATPase domain organization and proposed 2-gate mechanism of phospholipid translocation.
Most P4-ATPases are composed of a catalytic α subunit and a noncatalytic β subunit (Cdc50). Movements of the nucleotide binding (N), phosphorylation (P), actuator (A) and membrane domain helices during catalysis are modeled after SERCA1 structures trapped in the indicated conformational states. Clusters of residues that determine substrate specificity in TM1-4 labeled as the entry and exit gates. A substrate phospholipid is shown in green using the “Credit Card” model of transport to the cytosolic leaflet. Color figures can be found online.