Dear Editor,
In recent years, acute hepatopancreatic necrosis disease (AHPND) has rapidly spread in Asian countries and Mexico, causing severe mortality (up to 100%) and decreasing shrimp production.1, 2, 3, 4, 5, 6, 7, 8, 9 AHPND was originally shown to be caused by a specific virulent strain of Vibrio parahaemolyticus, namely the AHPND-causing V. parahaemolyticus (VPAHPND).1, 5, 6 V. parahaemolyticus becomes virulent VPAHPND after acquiring a plasmid (pVA1) expressing the deadly toxin PirVP, which consists of two subunits, PirA and PirB, and is homologous to the Pir (Photorhabdus insect-related) binary toxin.7 The plasmid pVA1 also carries a cluster of genes related to conjugative transfer; hence, this plasmid may potentially be able to transfer not only among V. parahaemolyticus strains but also to different bacterial species.7, 10 So far, there have been no published reports directly demonstrating that Vibrio campbellii can harbor pirVP and cause AHPND in shrimp. In this paper, we challenged Litopenaeus vannamei with a strain of V. campbellii (20130629003S01) carrying pirVP isolated from a L. vannamei farm and demonstrated that V. campbellii is a causative agent of AHPND.
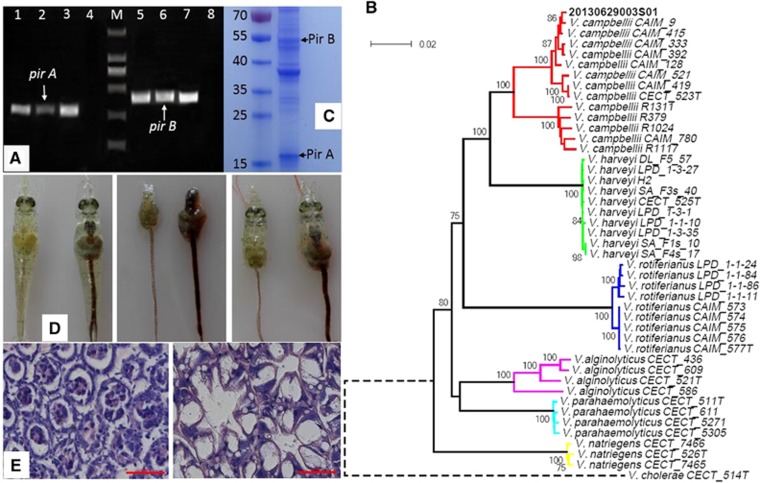
In this paper, strain 20130629003S01 was isolated in June of 2013 from diseased L. vannamei in Guangxi, China. PCR and RT-PCR amplifications were performed using VpPirA and VpPirB primers specific to pirVP genes (pirA and pirB).11 The electrophoresis of PCR products showed that both pirA (284 bp) and pirB (392 bp) were detected in the strain (Figure 1A). A partial sequence of 16S rRNA was obtained by sequencing the PCR products obtained with primers 27F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′-TAC GGC TAC CTT GTT ACG ACT T-3′).12 We found that strain 20130629003S01 belongs to the Vibrio core group, and its closest relatives are V. campbellii (99.72%) and V. rotiferianus (99.66%), according to the EzTaxon server (www.eztaxon-e.ezbiocloud.net). Partial sequences of σ70 factor (rpoD), replication initiator protein (rctB) and toxin transcriptional activator (toxR) were amplified as described by Pascual et al.13 After alignment of sequences for 16S rRNA and the rpoD, rctB and toxR genes, the phylogenetic tree was constructed with concatenated sequences by using neighbor-joining analysis in MEGA 5 (Tempe, AZ, USA). The multilocus sequence analysis clearly identified strain 20130629003S01 as being the closest to V. campbellii (Figure 1B).
Figure 1.
Identification and challenge tests of an isolate of Vibrio campbellii carrying pirVP genes associated with AHPND in Litopenaeus vannamei. (A) Detection of pirA and pirB genes. Lanes 1 and 5: PCR results from total DNA of strain 20130629003S01; lanes 2 and 6: RT-PCR results from extracted RNA of strain 20130629003S01; lanes 3 and 7: PCR results from purified plasmid DNA of strain 20130629003S01; lanes 4 and 8: non-template control; lane M: DL2000 DNA marker. (B) Phylogenetic reconstruction based on concatenated 16S rRNA, rpoD, rctB and toxR sequences. Percentage bootstrap values (1000 replicates) >75% are shown. Bar, 0.02 expected nucleotide substitutions per site. The reference sequences were as described by Pascual et al.13 The nucleotide sequences from strain 20130629003S01 have been submitted to the GenBank database under accession number KX534746 (16S rRNA), KX534747 (rpoD), KX534748 (rctB) and KX534749 (toxR). (C) SDS–polyacrylamide gel electrophoresis analysis of PirA and PirB from broth of strain 20130629003S01. (D) Gross signs of AHPND-infected shrimp (left): pale, atrophied hepatopancreas, empty stomach and gastrointestinal tract. Normal shrimp (right) in the negative control group: normal size hepatopancreas with brownish color and full stomach and gastrointestinal tract. (E) Hematoxylin and eosin-stained histological sections of the hepatopancreas of Litopenaeus vannamei from challenge tests. (Left) AHPND pathology characterized by sloughing of hepatopancreatic tubule epithelial cells. (Right) normal shrimp hepatopancreatic histology. Scale bars=50 μm. Abbreviation: acute hepatopancreatic necrosis disease, AHPND.
Significantly, pirVP genes were successfully amplified by using plasmid DNA extracted from the V. campbellii isolate (20130629003S01). In addition, next-generation sequencing of strain 20130629003S01 demonstrated that it also contains a pVA1-like plasmid containing pirVP (unpublished data). Protein profiles of the crude protein fractions of strain 20130629003S01 were analyzed as described by Sirikharin et al.14 Analysis by SDS–polyacrylamide gel electrophoresis revealed two target bands at marker levels of ~17 kDa (PirA) and 50 kDa (PirB; Figure 1C). Mass spectrometry analysis followed by the MASCOT analysis revealed that the two proteins had similarity to the published protein sequences of PirA (gi|922664586) and PirB (gi|922664588) of V. parahaemolyticus M0605.
The pathogenicity of strain 20130629003S01 was examined in healthy L. vannamei shrimps weighing ∼1 g, which were reared in 90 l artificial seawater at salinity 30 in plastic tanks (density 15 shrimps/tank) at 27±2 °C. An immersion challenge was used to follow the bioassay protocol described by Tran et al.5 All experimental groups were assayed in triplicate. Shrimp immersed with the bacterial suspension began to develop typical gross signs of AHPND within 12 h, massive mortalities occurred from 12 h post challenge, and cumulative mortalities reached 100% within 36 h. Gross signs of challenged L. vannamei included an empty stomach and gastrointestinal tract as well as pale and atrophied hepatopancreas (Figure 1D). A histopathological examination of moribund shrimp revealed the presence of AHPND lesions (Figure 1E) characterized by the acute sloughing of hepatopancreatic tubule epithelial cells, some of which displayed intact organelles, such as nuclei and cytoplasmic vesicles (Figure 1E). To our knowledge, our study is the first to demonstrate that a V. campbellii strain carrying pirVP causes AHPND. Therefore, AHPND caused by non-V. parahaemolyticus should be further investigated.
The shrimp farming industry is one of the important economic industries for countries in Asia and Latin America. AHPND is characterized by the acute and massive mortality in shrimp farms, causing severe production collapses and heavy economic losses. Ignoring the biosecurity of shrimp hatcheries and farms provides possibilities for the spread of VPAHPND. The existence of pirVP in non-V. parahaemolyticus isolates has been reported in a Vibrio harveyi-like stain from Vietnam15 and a Vibrio owensii-like from China.10 The present results may provide evidence for the horizontal transfer of the pirVP gene or pVA1 plasmid between different bacterial species, thereby potentially increasing the complexity of causative agents of AHPND and aggravating the threat to the shrimp industry. On the basis of our finding that a V. campbellii carrying pirVP causes AHPND, effective biosecurity measures should be considered to prevent the spread of AHPND in the future.
Acknowledgments
This work was supported by projects under the China Agriculture Research System (CARS-47), the Special Scientific Research Funds for Central Non-Profit Institutes, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (Grant No: 20603022016012), the Construction Programme for ‘Taishan Scholarship' of Shandong Province of China (S41201203) and the Project of the Aoshan Science and Technology Innovation Program of Qingdao National Laboratory for Marine Science and Technology (Grant No: 2015ASKJ02).
References
- Soto-Rodriguez SA, Gomez-Gil B, Lozano-Olvera R, Betancourt-Lozano M, Morales-Covarrubias MS. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei in Northwestern Mexico. Appl Environ Microbiol 2015; 81: 1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pena LD, Cabillon NA, Catedral DD et al. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Dis Aquat Organ 2015; 116: 251–254. [DOI] [PubMed] [Google Scholar]
- Gomez-Jimenez S, Noriega-Orozco L, Sotelo-Mundo RR et al. High-quality draft genomes of two Vibrio parahaemolyticus strains aid in understanding acute hepatopancreatic necrosis disease of cultured shrimps in Mexico. Genome Announc 2014; 2: e00800–e00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schryver P, Defoirdt T, Sorgeloos P. Early mortality syndrome outbreaks: a microbial management issue in shrimp farming? PLoS Pathog 2014; 10: e1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Nunan L, Redman RM et al. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ 2013; 105: 45–55. [DOI] [PubMed] [Google Scholar]
- Zhang BC, Liu F, Bian HH, Liu J, Pan LQ, Huang J. Isolation, identification, and pathogenicity analysis of a Vibrio parahaemolyticus strain from Litopenaeus vannamei (Chinese J.). Prog Fishery Sci 2012; 33: 56–62. [Google Scholar]
- Lee CT, Chen IT, Yang YT et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci USA 2015; 112: 10798–10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan L, Lightner D, Pantoja C, Gomez-Jimenez S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis Aquat Organ 2014; 111: 81–86. [DOI] [PubMed] [Google Scholar]
- Kondo H, Tinwongger S, Proespraiwong P et al. Draft genome sequences of six strains of Vibrio parahaemolyticus isolated from early mortality syndrome/acute hepatopancreatic necrosis disease shrimp in Thailand. Genome Announc 2014; 2: e00221–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Xiao J, Xia X, Pan Y, Yan S, Wang Y. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announc 2015; 3: e01395–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JE, Tang KF, Tran LH, Lightner DV. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis Aquat Organ 2015; 113: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard PP, Santini Y, Gruter D, Stettler R, Bachofen R. Bacterial diversity and community composition in the chemocline of the meromictic alpine Lake Cadagno as revealed by 16S rDNA analysis. FEMS Microbiol Ecol 2000; 31: 173–182. [DOI] [PubMed] [Google Scholar]
- Pascual J, Macian MC, Arahal DR, Garay E, Pujalte MJ. Multilocus sequence analysis of the central clade of the genus Vibrio by using the 16S rRNA, recApyrHrpoDgyrBrctB and toxR genes. Int J Syst Evol Microbiol 2010; 60: 154–165. [DOI] [PubMed] [Google Scholar]
- Sirikharin R, Taengchaiyaphum S, Sanguanrut P et al. Characterization and PCR detection of Binary, Pir-Like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in Shrimp. PloS one 2015; 10: e0126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Van PT, Dang LT, Hirono I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc 2015; 3: e00978–15. [DOI] [PMC free article] [PubMed] [Google Scholar]