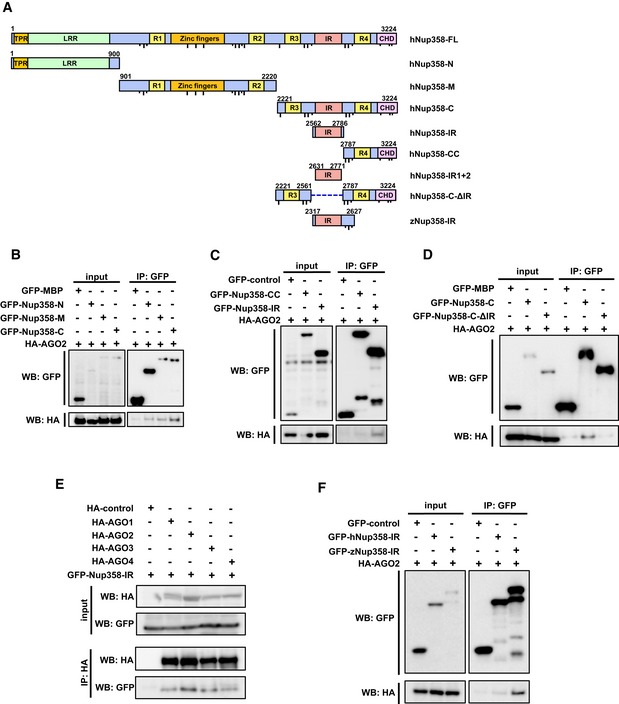
Schematic diagram representing the domains and constructs of human (h) and zebrafish (z) Nup358 used in this study. TPR, tetratricopeptide repeat; LRR, leucine‐rich region; R1–R4, RanGTP‐binding domain; ZnF, zinc finger domains; IR, internal repeats; CHD, cyclophilin‐homology domain. Dotted line indicates the deleted region in the indicated construct. FG and F × FG sequence positions are represented as short and long black lines, respectively. Amino acid positions are indicated in numbers. FL, full‐length; N, amino‐terminal region; M, middle region; C, carboxy‐terminal region.
HEK293T cells were co‐transfected with GFP‐maltose binding protein (MBP) control or GFP‐tagged version of indicated Nup358 fragments along with HA‐AGO2. Immunoprecipitation (IP) was performed using GFP antibodies and the presence of AGO2 was detected by Western blotting (WB) using HA antibodies.
Cells were transfected with indicated constructs and IP and WB were performed as described for (B).
HEK293T cells transfected with GFP‐MBP (control), GFP‐Nup358‐C, or GFP‐Nup358‐CΔIR (a mutant devoid of IR region) and HA‐AGO2. IP and WB analyses were performed as indicated.
Cells were transfected with HA‐tagged version of indicated AGO subfamily member and GFP‐Nup358‐IR. IP and WB analyses were performed using indicated antibodies.
Lysates from cells expressing GFP‐control, GFP‐human (h) Nup358‐IR, or GFP‐zebrafish (z) Nup358‐IR and HA‐AGO2 were immunoprecipitated using GFP antibodies, and the immunoprecipitates were analyzed for the presence of HA‐AGO2 by Western blotting.