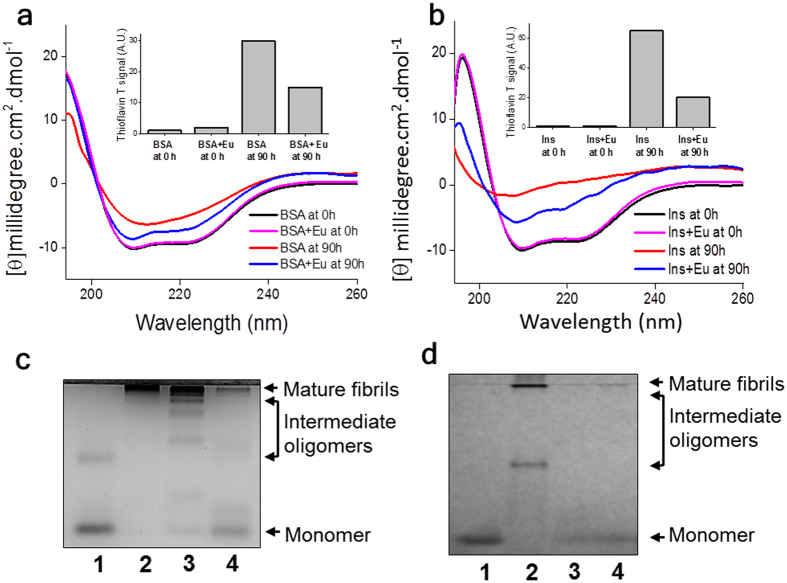
Figure 3. Structural studies on eugenol-protein interaction.
(a) CD spectra of BSA undergoing aggregation in the presence and in the absence of eugenol (at molar ratio of 1:400 of protein:ligand): BSA at 0 h ( ); BSA + eugenol at 0 h (
); BSA + eugenol at 0 h ( ); BSA at 90 h (
); BSA at 90 h ( ); BSA + eugenol at 90 h (
); BSA + eugenol at 90 h ( ). (b) CD spectra of insulin undergoing aggregation in the presence and absence of eugenol (at molar ratio of 1:100 of protein:ligand): insulin at 0 h (
). (b) CD spectra of insulin undergoing aggregation in the presence and absence of eugenol (at molar ratio of 1:100 of protein:ligand): insulin at 0 h ( ); insulin + eugenol at 0 h (
); insulin + eugenol at 0 h ( ); insulin at 90 h (
); insulin at 90 h ( ); insulin + eugenol at 90 h (
); insulin + eugenol at 90 h ( ). Insets of panel a and panel b show Thioflavin data for the respective CD samples. (c) Native gel-electrophoresis of the BSA (~5 μM) undergoing amyloid formation in the presence and in the absence of eugenol (at ~3 mM): (1) soluble BSA; (2) mature BSA aggregates; (3) BSA in the absence of eugenol at 5 h; (4) BSA in the presence of eugenol at ~5 h. (d) Native gel-electrophoresis of the insulin (~43 μM) undergoing amyloid formation in the presence and absence of eugenol at 1:150 molar ratio of insulin:inhibitor (1) soluble insulin; (2) mature insulin aggregates; (3) insulin in the absence of eugenol at ~6 h (4) insulin in the presence of eugenol at ~6 h. Uncropped native gel images are shown in Supplementary Figure S14.
). Insets of panel a and panel b show Thioflavin data for the respective CD samples. (c) Native gel-electrophoresis of the BSA (~5 μM) undergoing amyloid formation in the presence and in the absence of eugenol (at ~3 mM): (1) soluble BSA; (2) mature BSA aggregates; (3) BSA in the absence of eugenol at 5 h; (4) BSA in the presence of eugenol at ~5 h. (d) Native gel-electrophoresis of the insulin (~43 μM) undergoing amyloid formation in the presence and absence of eugenol at 1:150 molar ratio of insulin:inhibitor (1) soluble insulin; (2) mature insulin aggregates; (3) insulin in the absence of eugenol at ~6 h (4) insulin in the presence of eugenol at ~6 h. Uncropped native gel images are shown in Supplementary Figure S14.