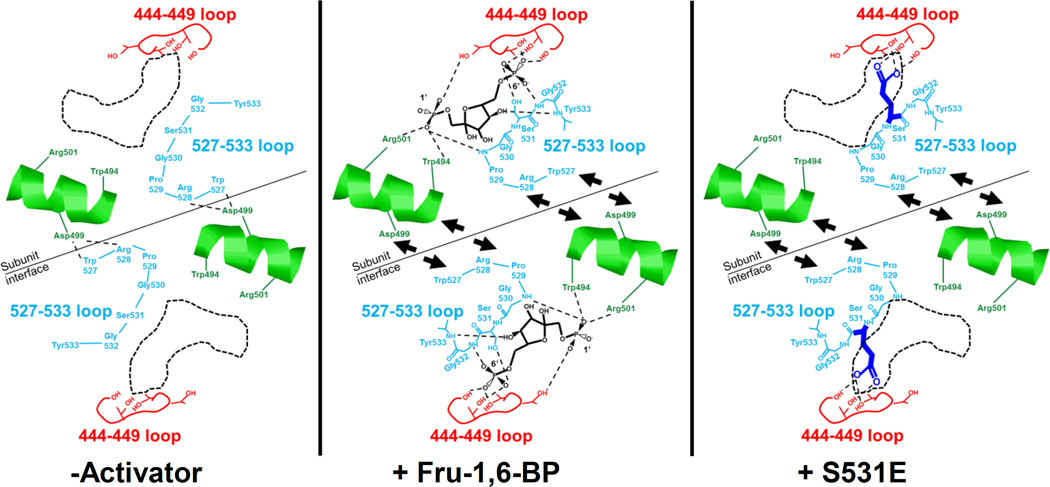
Figure 5.
A schematic of proposed allosterically relevant structural changes that are based on available PYK structures and that could be consistent with data in this study. In the absence of activator, Arg528 and Trp527 from the 527–533 loop of one subunit make contacts with Asp499 from the neighboring subunit. Upon Fru-1,6-BP binding or in the presence of the S531E mutation, the 527–533 loop is pulled away from the subunit interface. Interruption of the Trp527/Arg528/Asp499 interactions appears to be the primary requirement for allosteric activation. Movement of the helix containing Trp494, Asp499, and Arg501 may further relay the presence/absence of the Trp527/Arg528/Asp499 interactions to modify PEP affinity.