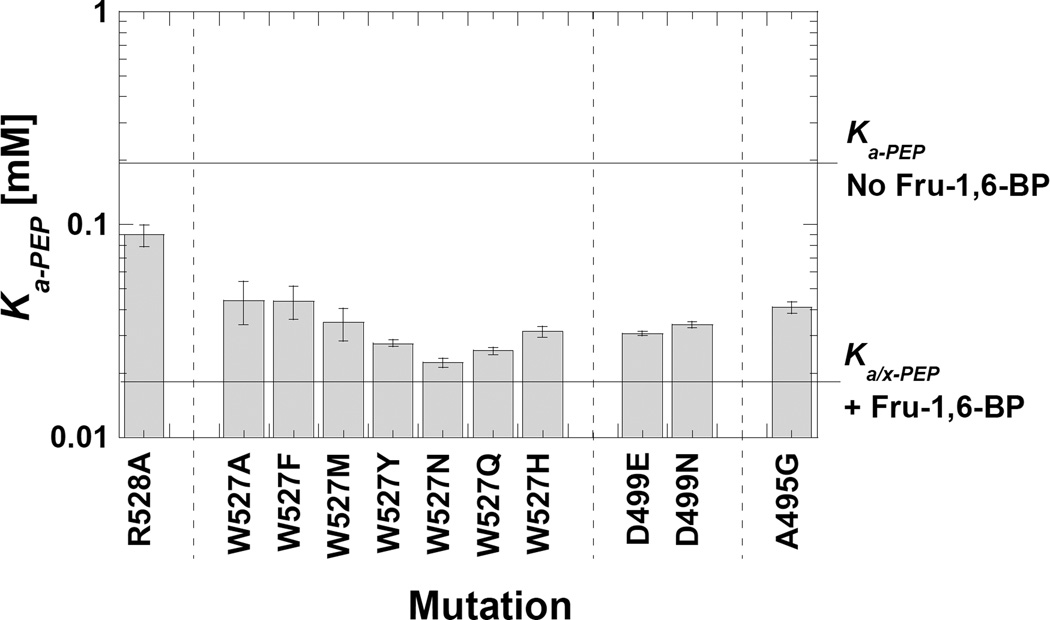
Figure 7.
Ka-app values for point mutations introduced with the intent of interrupting the Trp527/Arg528/Asp499 subunit interface interaction. The Ka-app values for the wild type proteins, both in the presence and absence of Fru-1,6-BP are indicated by horizontal reference lines. V498G and D499T mutant proteins were not activity and are not represented in the graph. Modification of Trp527 and Asp499 both caused increased affinity for PEP, similar to the allosteric activation by Fru-1,6-BP.