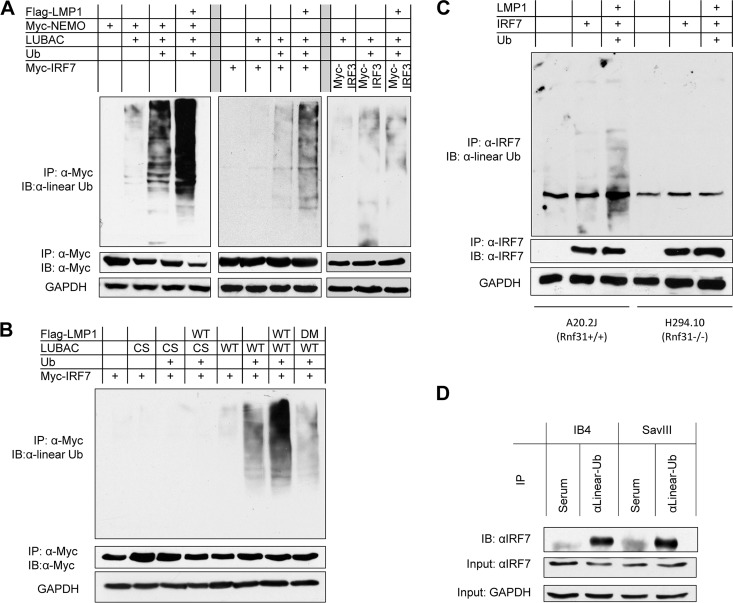
FIG 2.
LUBAC promotes linear ubiquitination of NEMO and IRF7. (A and B) 293T cells in 60-mm dishes were transfected with 0.5 μg Flag-LMP1, 0.5 μg Myc-NEMO or Myc-IRF7, 0.5 μg Ub, and 0.5 μg LUBAC (mixed with its three components). Cell lysates were prepared after 48 h with NP-40 lysis buffer. IP was performed with 1.5 μg anti-Myc 9E10 (Roche) and protein A/G-Sepharose beads (Santa Cruz) for 4 h. The beads were washed four times in NP-40 lysis buffer and two times with low-salt buffer (20 mM Tris, pH 7.4, 25 mM NaCl, 1 mM dithiothreitol [DTT]) and then denatured in 50 μl of 1% SDS. After dilution with buffer A (20 mM Tris, pH 7.4, 250 mM NaCl, 1 mM DTT, 1 mM sodium orthovanadate, 2 mM EDTA, 1% Triton X-100), the proteins were subjected to a second IP with 1 μg anti-Myc and protein A/G beads overnight. The beads were washed four times with buffer A and two times with low-salt buffer before immunoblotting with the linear ubiquitin antibody 1E3 (Millipore). (C) A20.2J (Rnf31+/+) and H294.10 (Rnf31−/−) cells were transfected with LMP1, IRF7, and Ub expression plasmids using a Nucleofector kit, following the manufacturers' instructions. Denatured immunoprecipitation was performed with anti-IRF7 G-8 (Santa Cruz), and then the immunoprecipitate was subjected to immunoblotting with the linear Ub antibody. (D) Cell lysates from IB4 and SavIII cells were denatured, precleared with rabbit serum, and then subjected to IP with rabbit anti-linear Ub or rabbit serum (control). After extensive washes, the beads were probed with rabbit anti-IRF7 (H-246). WT, wild type.