Figure 2.
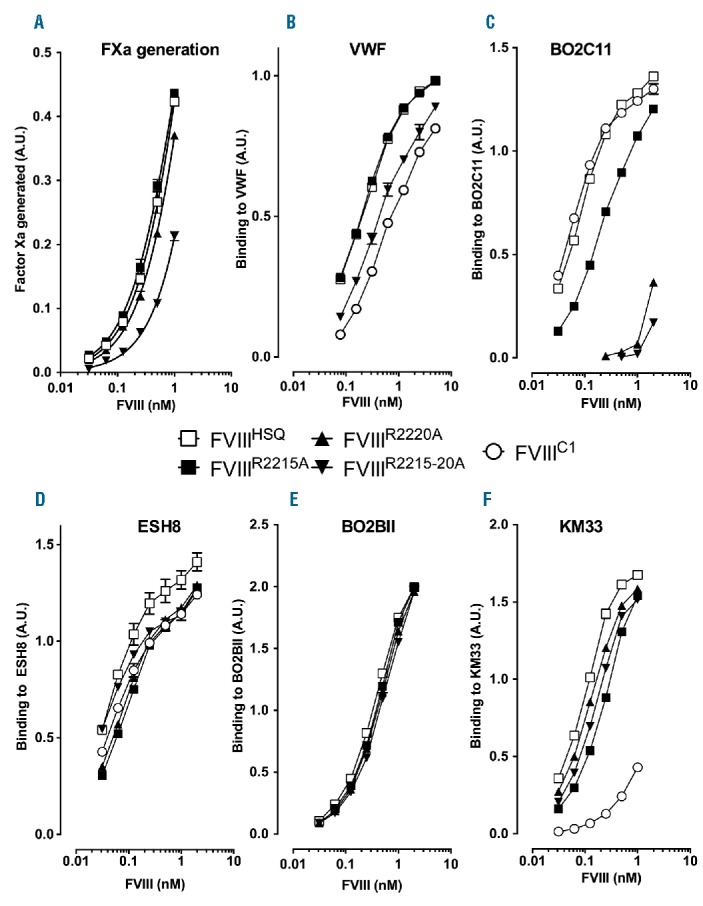
Characterization of FVIII containing alanine substitutions of the residues predicted to interact with BO2C11. Panel A. B domain-deleted wild-type FVIII (FVIIIHSQ), FVIIIR2215A or FVIIIR2220A were serially diluted 2-fold starting at 1 nM. Factor Xa generation was measured using a FVIII chromogenic assay. The reaction was stopped at 10 min using 20% acetic acid and the final absorbance measured at 405 nm. The data are represented as factor Xa generated in arbitrary units and are equivalent to the absorbance at 405 nm. VWF (Panel B), BO2C11 (human anti-C2 domain antibody, Panel C), ESH8 (mouse anti-C2 domain antibody that does not compete with BO2C11, Panel D), BO2BII (human anti-A2 domain antibody, Panel E) and KM33 (human anti-C1 domain antibody, Panel F) were immobilized on microtiter plates. After blocking, FVIIIHSQ, FVIIIR2215A, FVIIIR2220A, FVIIIR2215-20A or FVIIIC1 were serially diluted across the plates, and bound FVIII was revealed using either the biotinylated anti-A2 antibody, GMA-8015, or, in the case of BO2BII-bound FVIII, ESH8, followed by an anti-mouse-HRP antibody. The graphs are represented as absorbance measured at 492 nm (mean±SEM). FXa: factor Xa; VWF: von Willebrand factor; A.U.: arbitrary unit; FVIII: factor VIII.
