Figure 4.
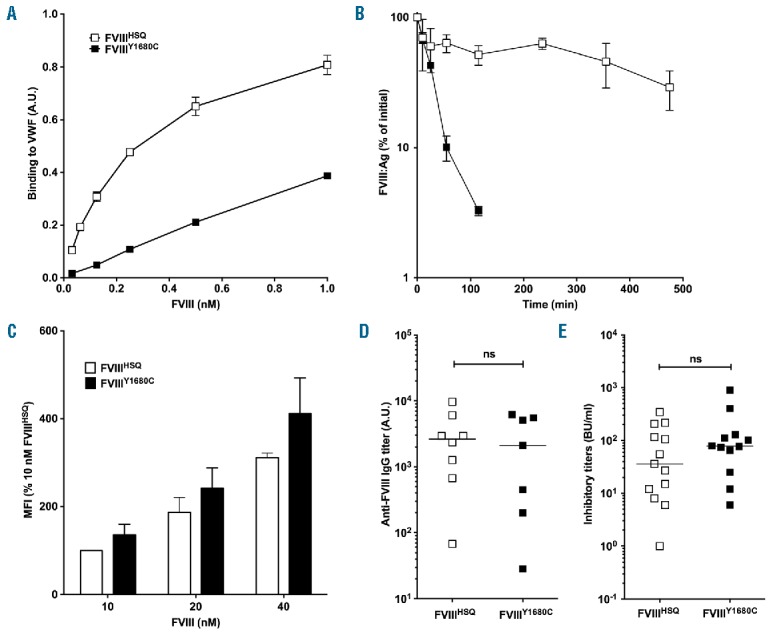
FVIII binding to VWF does not alter endocytosis in vitro or immunogenicity in vivo. Panel A. The binding of B domain-deleted FVIIIHSQ and FVIIIY1680C to VWF was studied using an ELISA as described in the Methods. Results (mean±SEM) are expressed in arbitrary units. Panel B. FVIIIHSQ (10 nM, open squares) or FVIIIY1680C (closed squares) in 100 μl were administered into FVIII-deficient mice and the residual FVIII levels were measured at different time points (n=3 mice per time point) by ELISA (FVIII:Ag). The data is plotted as a percentage of the initial FVIII level (measured 5 minutes after administration, mean±SEM) versus time. Panel C. FVIIIHSQ or mutants FVIIIY1680C were added at increasing concentrations (10, 20 and 40 nM) to immature MoDCs (n=3 different donors) for 30 min at 37°C in serum-free medium and in the absence of VWF. Cells were subsequently washed, fixed, permeabilized and stained for FVIII using a FITC-labeled anti-A2 domain-specific antibody followed by fluorescence-activated cell sorting (FACS). Data are expressed as the percentage of MFI (mean±SEM), whereby 100% corresponds to the MFI observed for 10 nM of FVIIIHSQ. Panels D and E. FVIII-deficient mice were administered with FVIIIHSQ (0.5 μg, open squares) or FVIIIY1680C (0.5 μg, closed squares), intravenously once a week for 4 weeks. Anti-FVIII IgG titers were measured using an ELISA as described above (D). Inhibitory titers towards FVIII were assessed using a modified Bethesda assay (E). Horizontal bars depict medians. The statistical significance was assessed using the two-tailed non-parametric Mann-Whitney U test. ns: not significant; FVIII: factor VIII; VWF: von Willebrand factor; A.U.: arbitrary unit; MFI: median fluorescence intensity; IgG: immunoglobulin G; Ag: antigen.
