Tyrosine kinase inhibitors (TKI) provide an efficient ‘targeted’ therapy against the constitutively expressed BCR-ABL1 oncoprotein characterizing chronic myeloid leukemia (CML). Due to its success in adult CML patients, the continuing treatment with TKI, namely imatinib, has also replaced allogeneic stem cell transplantation in pediatric patients as front-line therapy.1
Tight molecular monitoring of tumor load reveals that imatinib monotherapy induces a biphasic decline of BCR-ABL1 transcript levels in most adult CML patients. While the initial steep decline most likely results from the rapid depletion of actively cycling BCR-ABL1 positive cells, the second moderate decline may represent the slow elimination of quiescent residual leukemic stem cells owing to their comparatively low turnover.2 However, CML is rare in cohorts of patients under 20 years of age, and data on the kinetics of the BCR-ABL1 expression in response to TKI treatment in children and teenagers are still scarce. While it is widely agreed that the cellular and molecular features of CML in children are identical to adults, it must be remembered that the host is still a growing organism,3 and initial tumor cell burden and treatment responses may vary according to age.4,5
Here, we provide the first comprehensive overview of the temporal, biphasic kinetics of BCR-ABL1 transcript reduction in a cohort of pediatric and teenage patients enrolled on the pediatric prospective CML-PAED II trial (clinicaltrials.gov identifier: 00445822) in response to a standardized up-front treatment with imatinib. In particular, we apply a bi-exponential regression model to parameterize the clinical response that is used to compare the pediatric cohort to adult CML patients.
Eighty-seven patients (age 1–18 years) with a diagnosis of CML in chronic phase (CP) enrolled on the prospective, international CML-PAED-II trial during the recruitment period from 2006 to 2012 were available for our study. For detailed analysis, out of these 87 patients, we included only 40 national cases for whom nested PCR measurements were available in case of qPCR negativity. Written informed consent was obtained from all patients or their legal guardians according to the Declaration of Helsinki. The study was approved by the Ethical Committee of the Medical Faculty of the Technische Universität Dresden, Germany (ethical vote #EK282122006).
All pediatric patients received standard treatment with imatinib 260–340 mg/m2 within a week after diagnosis of CML had been confirmed by either cytogenetic or molecular analysis. No other cytostatic treatment prior or in addition to imatinib was administered. Therapeutic response was monitored by measuring the BCR-ABL1/ABL1 transcript ratio in blood specimens, typically at 1, 2, and 3 months and subsequent intervals of 3–6 months after commencing imatinib, using a standardized approach for molecular diagnosis of CML by qRT-PCR. Measurements were performed and results reported according to the International Scale (IS).6 In case of BCR-ABL1 negativity by qRT-PCR, nested PCR was performed. Forty-one percent of the specimens that had tested negative by qRT-PCR showed positive results using a nested PCR approach. For the numerical analysis, we assigned a plausible lower approximation of the detection threshold at MR5 (undetectable BCR-ABL1 in >100,000 ABL1 transcripts7) to all the negative results, which were further used for the calculation of medians and individual responses, as well as for the graphical visualization of time courses.
For statistical analysis of treatment response, a minimal data set of 7 or more consecutive BCR-ABL1 level measurements was required over a follow-up interval of over one year. Early non-responders were characterized by a BCR-ABL1/ABL1 of more than 10% after 18 months of treatment and were also excluded from the analysis, leaving 35 out of 40 patients for further analysis. Biphasic decline kinetics of BCR-ABL1 levels in response to imatinib were sufficiently described by a bi-exponential regression model:8
Here, parameter α characterized the slope of the first, steeper decline kinetics, whereas parameter β characterized the slope of the second, moderate decline kinetics (Figure 1A). Parameters A and B could be interpreted as the corresponding (approximate) intercepts with the y-axis at time point 0 (=treatment start). The breakpoint between first and second slope was characterized by its time of occurrence (timebp) and its corresponding BCR-ABL1/ABL1 level (ratiobp). Furthermore, the Davies test was used to test for a non-constant regression parameter in the linear predictor, i.e. whether the slopes α and β differ.9 Out of the 35 patients with sufficient follow up (>7 measurements), 25 fulfilled this criterion of a biphasic decline in BCR-ABL1. Time courses of all patients are available in the Online Supplementary Figures.
Figure 1.
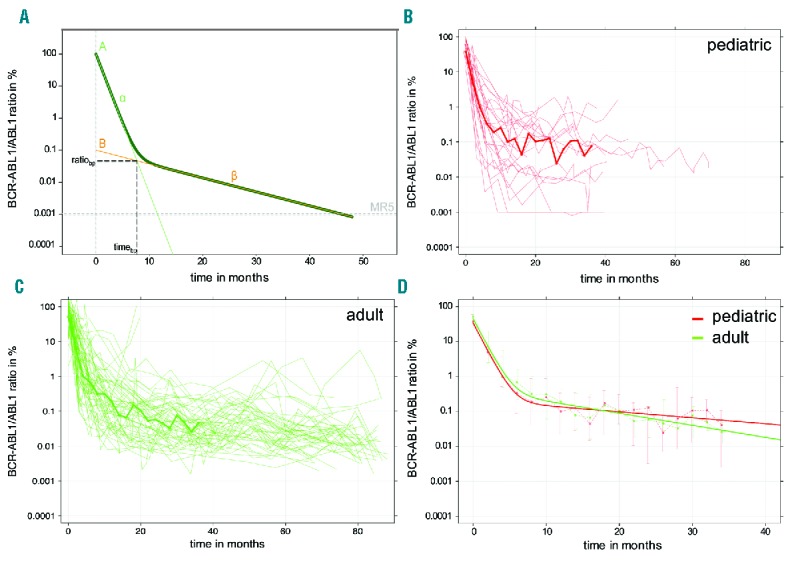
Individual and median time courses. (A) The statistical bi-exponential model describes the patient-specific treatment response by a ‘two slope dynamic’, in which the slope of the first decline is denoted as α and the slope of the second moderate decline as β. The breakpoint between first and second slope is characterized by its time of occurrence (timebp) and its corresponding BCR-ABL1/ABL1 level (ratiobp). (B and C) Individual time courses for all (B) pediatric (n=25) and (C) adult (n=55) patients. Solid lines indicate median values of all patients for whom BCR-ABL1/ABL1 ratios are available within 2-month intervals, (D) Comparison of the response kinetics using the bi-exponential regression model (solid lines), which is fitted to the median responses of the pediatric and adult patient cohorts. Whiskers indicate upper and lower quartiles.
For comparison with adult data, we used a cohort of 69 patients from the German cohort of the IRIS trial.10 Applying the same selection criteria, 62 patients had a sufficiently long follow up and 55 of them followed a biphasic decline characteristic. We used Wilcoxon tests to test for differences in the distribution of treatment parameters of both cohorts using software R for statistical analysis (v.3.2.0; www.r-project.org).
For the analysis of typical biphasic response kinetics, time courses from the 25 pediatric patients (male/female: 16/9; median age 11.9 years, range 4.5–17.6) were compared to 55 adult patients (male/female 40/15; median age 52.5 years, range 21–69). Time courses for these subpopulations are shown in Figure 1B and C. Obviously, follow up in the pediatric cohort was much shorter than in the adult cohort (median follow up time 30.6 months for pediatric patients vs. 79.1 months in adult patients).
To quantitatively compare the cohorts, we took the median values of the available measurements for 2-month intervals and derived a ‘median response’ (shown as a bold line in Figure 1B and C). As the number of measurements in the pediatric cohort dropped substantially after three years, we limited the time interval for this analysis to 36 months. For the resulting median response, we fitted a bi-exponential regression model, thus obtaining A, B, α and β as the characteristic parameters for the mean response in each cohort (Table 1 and Figure 1D).
Table 1.
Statistical parameters of median response characteristics of pediatric and adult patients.
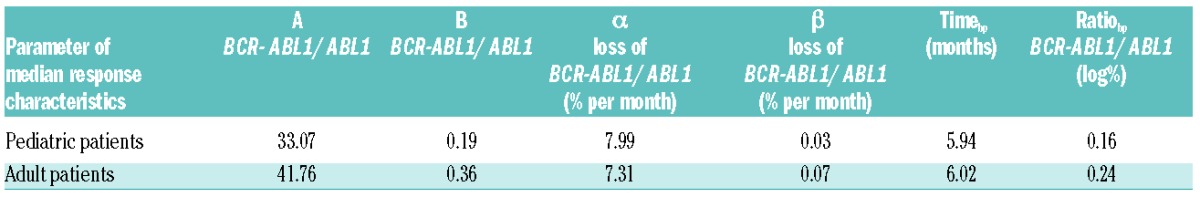
On average, the BCR-ABL1/ABL1 transcript levels initially declined with a rate of 8% per month in the pediatric cohort and 7.3% per month in the adult cohort (α slope) (Table 1). This minor difference should be interpreted with caution, as there is a known inaccuracy of BCR-ABL1/ABL1 measurements of over 10%. Moreover, this effect was compensated by a smaller decline within the second slope (0.03% vs. 0.07% per month). There appeared no distinct differences in the median response kinetics between pediatric and adult patients (Figure 1D). In order to address the statistical significance of these findings, we focused on the kinetics for each individual patient.
Next we applied a bi-exponential regression model for each individual patient, thereby obtaining a distribution of individual decline parameters (namely A, B, α, β, timebp, ratiobp) for both cohorts. Histograms in Figure 2 indicate that the breakpoint of the response kinetics typically occurred between 2 and 11 months (Figure 2E), while the BCR-ABL1/ABL1 level of the breakpoint was typically below 1% (Figure 2F). Distribution of the decline parameters (A, B, α, β) between pediatric and adult patients appeared to be very similar. A comparison of the medians by Wilcoxon test (see P-values in Figure 2) only detected a statistically significant difference for the initial values of log(A), which roughly corresponded to the BCR-ABL1/ABL1 level detected at diagnosis (P=0.007). However, as mentioned above, caution is warranted as the use of ABL1 as a reference gene of the PCR-reaction biases the results for high values of BCR-ABL1/ABL1.11
Figure 2.
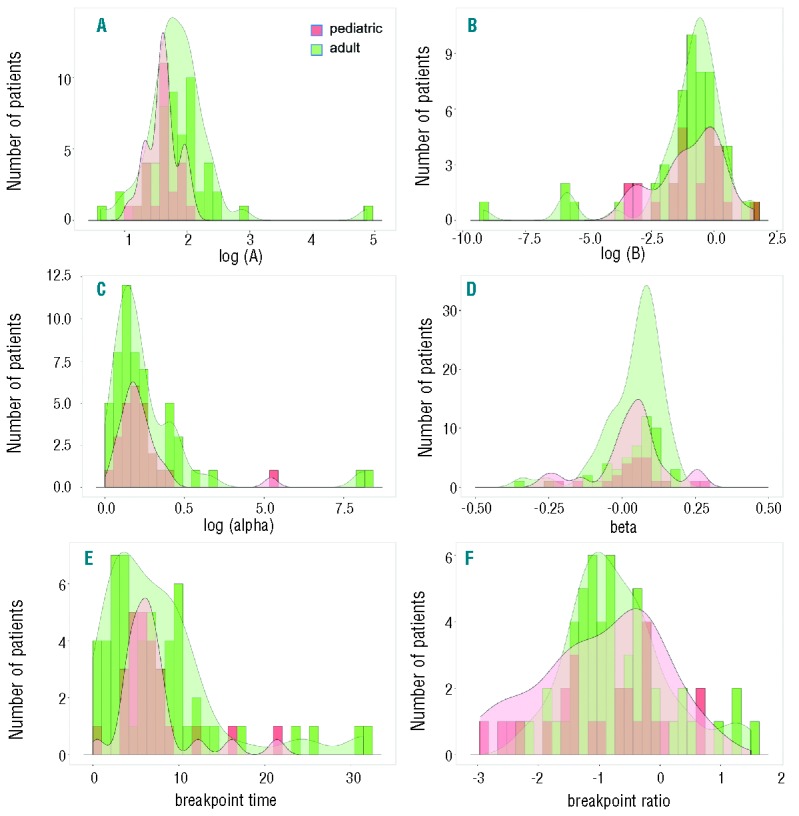
Comparison of the individual parameters of the segmented regression model. Histograms (pediatric: red; adult: green) with overall density kernels (shaded) for the parameters of the statistical model: (A) intercept A [shown as log(A), Wilcoxon test P=0.007]; (B) intercept B [shown as log(B), P=0.950]; (C) slope α [shown as log(α), P=0.909]; (D) slope β (P=0.506); (E) time of breakpoint timebp in months (P=0.803); (F) level at breakpoint ratiobp (P=0.455).
Furthermore, we did not find any evidence that age at diagnosis or the presence of an e13a2/e14a2 splicing variant of the BCR-ABL1 oncogene has an influence on the individual patient responses.
Our statistical analysis of primary kinetic responses of pediatric CML patients treated with the TKI imatinib revealed no distinct differences from a reference adult cohort. Apart from some patients showing a uniphasic decline in BCR-ABL1 ratios, the majority of the patients showed the typical biphasic response (25 of 35 in the pediatric and 55 of 69 in the adult cohort). Overall, there was a slightly higher fraction of pediatric patients achieving MR4 already during the initial decline (3 of 40 children vs. 1 of 69 adults). Although several studies suggest that initial response to imatinib treatment was inferior in e13a2 patients,3 we found no statistically relevant differences in treatment response between patients harboring the e13a2 and the e14a2 phenotype of CML. However, given the small sample size (which is approx. a factor 10 smaller than the relevant studies in adult patients) this effect may have been been masked.
Tyrosine kinase inhibitor treatment in children inhibits not only BCR-ABL1, but also exerts many side effects, such as dysregulation of bone remodeling,12 longitudinal growth impairment,13,14 impaired vitamin D synthesis,15 growth hormone deficiency,16 and other metabolic dysregulations. As these side effects may become more significant with longer periods of TKI treatment, sophisticated approaches to support the safety of TKI therapy cessation, such as model-based risk estimations, are even more desirable in pediatric cohorts than for adult patients.
Supplementary Material
Acknowledgments
The authors wish to thank Matthias Horn for valuable advice and critical discussions.
Footnotes
Funding: this work was supported by the German Federal Ministry of Research and Education, Grant number 031A315 “MessAge” and Grant number 031A424 “HaematoOpt”.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.de la Fuente J, Baruchel A, Biondi A, et al. Managing children with chronic myeloid leukaemia (CML): recommendations for the management of CML in children and young people up to the age of 18 years. Br J Haematol. 2014;167(1):33–47. [DOI] [PubMed] [Google Scholar]
- 2.Stein AM, Bottino D, Modur V, et al. BCR-ABL transcript dynamics support the hypothesis that leukemic stem cells are reduced during imatinib treatment. Clin Cancer Res. 2011;17(21):6812–6821. [DOI] [PubMed] [Google Scholar]
- 3.Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2015;127(4):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalmanti L, Saussele S, Lauseker M, et al. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomized CML study IV. Ann Hematol. 2014;93(1):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pemmaraju N, Kantarjian H, Shan J, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97(7):1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller MC, Cross NC, Erben P, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23(11):1957–1963. [DOI] [PubMed] [Google Scholar]
- 7.Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein AM, Martinelli G, Hughes TP, et al. Rapid initial decline in BCR-ABL1 is associated with superior responses to second-line nilotinib in patients with chronic-phase chronic myeloid leukemia. BMC Cancer. 2013;3:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies RB. Hypothesis testing when a nuisance parameter is present only under the alternative. Biometrika. 1987;74(1):33–43. [Google Scholar]
- 10.Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010; 116(19):3758–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanfstein B, Lauseker M, Hehlmann R, et al. Distinct characteristics of e13a2 versus e14a2 BCR-ABL1 driven chronic myeloid leukemia under first-line therapy with imatinib. Haematologica. 2014; 99(9):1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandyke K, Fitter S, Dewar AL, Hughes TP, Zannettino AC. Dysregulation of bone remodeling by imatinib mesylate. Blood. 2010;115(4):766–774. [DOI] [PubMed] [Google Scholar]
- 13.Bansal D, Shava U, Varma N, Trehan A, Marwaha RK. Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediat Blood Cancer. 2012;59(3):481–484. [DOI] [PubMed] [Google Scholar]
- 14.Tauer JT, Nowasz C, Sedlacek P, et al. Impairment of Longitudinal Growth By Tyrosine Kinase Inhibitor (TKI) Treatment - Data from a Large Pediatric Cohort with Chronic Myeloid Leukemia (CML). Blood. 2014;124(21):522. [Google Scholar]
- 15.Mehlig LM, Garve C, Tauer JT, Suttorp M, Bauer A. Inhibitory effects of imatinib on vitamin D(3) synthesis in human keratinocytes. Mol Med Rep. 2015;11(4):3143–3147. [DOI] [PubMed] [Google Scholar]
- 16.Giona F, Mariani S, Gnessi L, et al. Bone metabolism, growth rate and pubertal development in children with chronic myeloid leukemia treated with imatinib during puberty. Haematologica. 2013; 98(3):e25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


