Abstract
The role of lactic acid bacteria (LAB) in honey as antifungal activity has received little attention and their mechanism of inhibitory of fungi is not fully understood. In this study, LAB were isolated from honey samples from Malaysia, Libya, Saudi Arabia, and Yemen. Twenty-five isolates were confirmed LAB by catalase test and Gram staining, and were screened for antifungal activity. Four LAB showed inhibitory activity against Candida spp. using the dual agar overlay method. And they were identified as Lactobacillus plantarum HS isolated from Al-Seder honey, Lactobacillus curvatus HH isolated from Al-Hanon honey, Pediococcus acidilactici HC isolated from Tualang honey and Pediococcus pentosaceus HM isolated from Al-Maray honey by the 16S rDNA sequence. The growth of Candida glabrata ATCC 2001 was strongly inhibited (>15.0 mm) and (10~15 mm) by the isolates of L. curvatus HH and P. pentosaceus HM, respectively. The antifungal activity of the crude supernatant (cell free supernatant, CFS) was evaluated using well diffusion method. The CFS showed high antifungal activity against Candida spp. especially The CFS of L. curvatus HH was significantly (p < 0.05) inhibited growth of C. glabrata ATCC 2001, C. parapsilosis ATCC 2201, and C. tropicalis ATCC 750 with inhibitory zone 22.0, 15.6, and 14.7 mm, respectively. While CFS of P. pentosaceus HM was significantly (p < 0.05) effective against C. krusei, C. glabrata, and C. albicans with inhibition zone 17.2, 16.0, and 13.3 mm, respectively. The results indicated that LAB isolated from honey produced compounds which can be used to inhibit the growth of the pathogenic Candida species.
Keywords: Antifungal activity, Honey, Lactic acid bacteria, Pathogenic Candida species
A supersaturated four key sugars solution of fructose, glucose, sucrose and maltose, honey has other elements for example gluconic acid, protein enzymes, amino acid, minerals, vitamins, and water [1]. Slightly acidic with a pH between 3.2 and 4.5, honey's low pH prevents the growth of various pathogenic bacteria and fungi [2]. Honey's antimicrobial activity is affected by how microbial groups including bacteria (Gram-variable pleomorphic bacteria, Bacillus spp., Enterobacteriaceae, and lactic acid bacteria [LAB]), molds (primarily aspergilli and penicillia) and yeasts interact with each other [3]. Honey's inherent elements might influence microorganisms' growth and ability to survive via bacteriostatic or bactericidal actions [4]. Comparable to fermented food and silage situation that is both rich in sugar and acid, LAB are honey's major microorganism [5], honey bee (Apis mellifera) foragers [6], bees' stomach, flowers, plants, flowers, and fruits [7]. LAB secluded from diverse bases is identified in yielding different antimicrobial compounds that are able to prevent fungi growth. Generally, some species inside LAB might yield bioactive compounds for instance organic acid, hydrogen peroxide, diacetyl, bacteriocins, antimicrobial peptides, and antibiotics [8,9,10].
Pathogenic Candida types are the commonest reason for hospital-acquired contagions particularly, among cancer and transplant patients who used immunosuppressive therapy. Candida species are behind various forms infecting humans for example urinary tract infection, vulvovaginal candidiasis, and nosocomial pneumonias [11]. Several types of Candida spp. are resistant to numerous non-natural antifungal drugs for example amphotericin B, fluconazole and itraconazole. These three antifungal agents are normally part of candidemia and invasive candidiasis treatment [11,12]. Several cases correlated to long term suppressive therapy using antifungal agents was reported to be futile and poisonous to the host [13,14]. Many patients experiencing fluconazole caused clinical failure will have Candida species separates to in vitro resisting antifungal [12]. Honey is natural, non-poisonous and robust antimicrobial can be a favorable substitute to antifungal agent. Samplings from honey antifungal activities are different based on their botanical source and geographical origin [15]. Earlier studies emphasized that honey from Algeria [16], Nigeria [17], and Turkey [18], exhibited antifungal activity, however, the studies did not examine honey's LAB role as an antifungal agent. Hence, this study's purpose is determining the LAB isolated antifungal activity from honey from different sources for example Malaysia, Libya, Yemen, Saudi Arabic, and New Zealand against Candida spp. strains specifically, C. albicans ATCC1405, C. glabrata ATCC2001, C. tropicalis ATCC750, C. parapsilosis ATCC22019 and C. krusei ATCC6258.
MATERIALS AND METHODS
Honey samples
The collection of fifteen samples of honey from various sources was stored at room temperature prior to analysis. This study's samples were honey locally attained from Malaysia (Madu Tualang, Madu Tani, pure Trigona honey) Libya (Al-Seder honey, Al-Hanon honey, and Al-Zater honey), Yemen (Al-Seder honey and Al-Maray honey), Saudi Arabic (Al-Shifaa honey), and New Zealand (Manuka honey). A pH meter (METTLER TOLEDO, Greifensee, Switzerland) was used in determining the diverse honey's pH range.
Isolation of LAB from honey samples
Isolation of LAB from honey samples succeeding the technique defined by Bulgasem et al. [19]. The suspension of roughly 10 g of honey samples in 90 mL peptone water (0.1% w/v) in sterile stomacher bags and the bags' agitation were manually done. Then, the addition of 1 mL into 9 mL of de Man, Rogosa & Sharpe (MRS) broth (Oxoid CM359) and the incubation was at 30℃ for 24 to 48 hr followed by diluting serially with peptone water (0.1% w/v). Subsequently, 0.1 mL was spread plated on a number of adapted media specifically, MRS agar (Oxoid) [20], MRS agar with 0.8% CaCO3 [21], MRS agar with 1% glucose, tomato juice agar with 0.8% CaCO3, and tomato juice agar with 1% glucose. The incubation of plates was under anaerobic situation in anaerobic jar at 37℃ for 48 hr or until the bacterial colonies grown sufficiently in size. The testing of colonies for catalase activity with 4% H2O2 and the streaking of catalase negative colonies on MRS agar that contained 0.8% CaCO3 was kept warm at 37℃ for 48 hr to attain pure colonies. The isolates' validation for Gram staining and culture purity was inspected using morphology and microscopic. All negative catalase and gram-positive LAB isolates were preserved in MRS broth with 15% of glycerol and set aside at −20℃ for more inspection.
Culturing of Candida spp
The Candida strains in this study were attained from microbial collections original stock cultures of Department of Medical Microbiology, University Putra Malaysia. All Candida spp. including C. albcans ATCC14053, C. parapsilosis ATCC22019, C. tropicalis ATCC750, C. krusei ATCC 6258, and C. glabrata ATCC2001 strains which were cultured on Sabouraud dextrose agar (SDA, Oxoid) at 35℃ for 24 and 48 hr to confirm feasibility and clarity. Fungal strains were kept on SDA at 4℃ until further use.
Cell free supernatant preparation
The isolates were injected into MRS broth and keep warm at 30℃ for 24 hr. The preparation of cell free supernatant (CFS) was by centrifuging the broth at 11,500 rpm for 10 min at 4℃. (Mini Spin, AG 22331; Eppendorf, Hamburg, Germany). Each filtration of isolate supernatant used sterile filter (0.45 µm-pore-size filter; Millipore, Darmstadt, Hesse, Germany) [22], and this filtrate was utilized for analysis.
Sensitivity of Candida spp. to antifungal agents
Candida spp. was examined to see how sensitive it is to antifungal agents via disc diffusion technique as defined by Bauer et al. [23]. The antifungal utilized were nystatin (100 U), amphotericine B (20 µg), fluconazole (100 µg), ketoconazole (10 µg), itraconazole (50 µg), voriconazole (10 µg), and econazole (10 µg). The antifungal disks were attained from SIGMA-Aldrich Chemie GmbH (Steinheim, Germany). Antifungal agents selected to be utilized in this study was established on the common antifungal utilized in medical practice and health therapy. Candida spp.'s multiple antifungal resistant (MAR) index was decided when the number of antifungal agents which is resistant to an isolate/whole quantity of antifungal tried as defined by Subramani and Vignesh [24]. Candida spp.'s 24-hr pure cultures cultured in Sabouraud dextrose broth (Oxoid CM147) and kept warm at 37℃ for 24 hr. The pathogenic Candida cultures were later swabbed on SDA (Oxoid) plates. The plates' agar surface were dried in the laminar flow cabinet at ambient temperature for 15 min. With a sterilized forceps, the antifungal agents' paper discs (6 mm in diameter) were aseptically placed on plates and the incubation was at 37℃ for 24 hr aerobically. The inhibitory zone's diameter measurement surrounding each disc was taken using a ruler and documented. The experiment was carried out two times and the mean and standard deviation was noted.
Antifungal activity of LAB isolates using dual agar overlay method
Four LAB isolates antifungal activities was decided against Candida spp. using the overlay technique as defined by Magnusson and Schnürer [25]. LAB was injected in spot on MRS agar plates. The incubation of the plates was at 30℃ for 24 hr under anaerobic situations. These plates were covered with a layer of 15mL of SDA (0.75% soft SD agar) that contained 104 CFU/mL of overnight Candida culture poured over the plates. The plates were kept warm aerobically at 30℃ for 24 hr, and the zone inhibiting Candida growth located above respective LAB culture was measured. LAB inhibition tests against Candida spp. was carried out in duplication.
Antifungal activity of LAB supernatants using agar well diffusion method
The LAB isolates exhibited strong antifungal activity via dual overlay spot technique was additionally assessed by the well technique defined by Magnusson and Schnürer [25]. Candida spp. (104 cells/mL) cultured 24 hr was diversified with SDA, and dispensed into the plates. After the solidification of agar, cork borer were used to make 6-mm wells. It then covered the well's base with agar to evade leaks. The addition of CFS invariable quantities of LAB to each well and incubating the plates aerobically at 30℃ for 24 hr. The measurement of growth inhibition zone surrounding the wells was taken after well size decreases. The experiment was carried out in duplicates and the means with calculations of standard deviations.
Identification of LAB isolates by API 50 CHL and 16S rDNA gene sequences
The four LAB isolates showing antifungal activity were recognized with API 50 CHL kit assay resulting from the technique used as described by the manufacturer [26]. The LAB isolates identified were further established by 16S rDNA gene sequences. The LAB chromosomal DNA's four strains were taken out using the Wizard Genomic gram-positive DNA purification kit (Promega, Madison, WI, USA). The sample's purified DNA was processed to the polymerase chain reaction (PCR) by utilising the Fail Safe Pre Mix Kit Epicentre (an Illumina company, San Diego, CA, USA). One thousand four hundred base pair fragment of 16S rDNA gene was amplified using PCR with primers based on the preserved 16S forward (5′-AGAGTTTGATCCTGGCTC-3′) and 16S reverse (5′-CGGGAACGTATTCAC-CG-3′) regions [27]. The PCR protocols were as follows: denaturation at 95℃ for 2 min, followed by 35 denaturation cycles at 92℃ for 45 sec, hardening at 54℃ for 1min and elongating at 72℃ for 1min, and final extension at 72℃ for 5min. Five microliters of each amplified mixture were exposed to electrophoresis in 1.5% (1.5 g agarose powder with 100 mL in 1× TEA buffer) for 45 min at 90 V. Amplified products were examined more by the First BASE Laboratories Sdn Bhd., Malaysia. The aligned nucleotide sequence was later exposed to the Nucleotide-nucleotide Basic Local Alignment Search Tool (BLASTN) software ver. 2.2.14 to be compared to other accessible isolates in the GenBank Database of the National Centre for Biotechnology Information (NCBI) to be confirmed. The multiple sequence alignment was done using Multiple Sequence Comparison by Log-Expectation (MUSCLE) [28], and phylogenetic tree was constructed by the neighbor-joining method [29]. Bacillus cereus ATCC14579 used as an out-group organism with 1,000 bootstrap replicates. The nucleotide sequences for the 16S rDNA described in this study were deposited with the GenBank database of Bethesda, MD, USA under accession Nos. KX346613~KX346616 for the strains HS, HC, HH, and HM.
Statistical analysis
All data were presented as mean ± standard deviation and were analysed by two-way analysis variance using general liner model procedure of SAS (SAS Institute Inc., Cary, NC, USA), and Tukey's test was applied for significant means at p < 0.05 to evaluate the significant differences between groups.
RESULTS
Screening of antifungal LAB
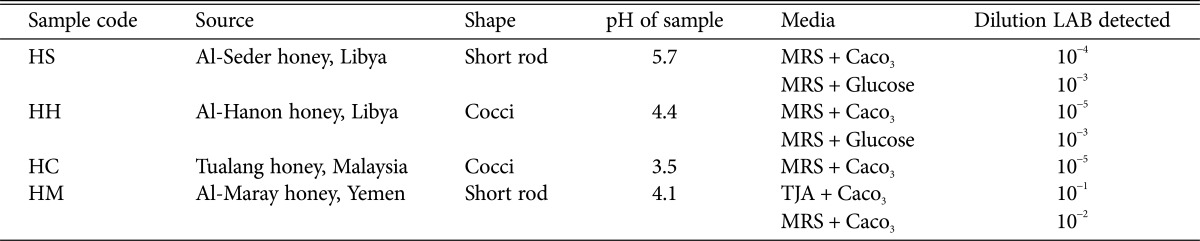
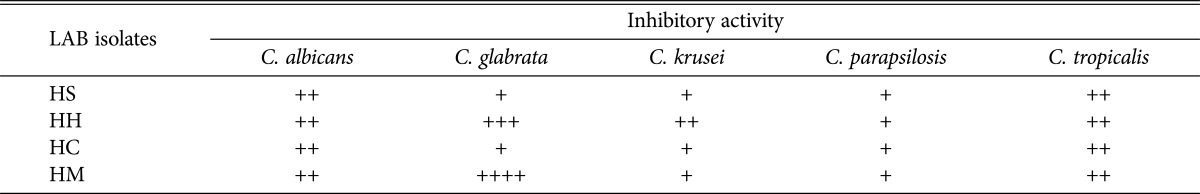
Isolation of LAB from honey was performed by using modified media. Among the different medium evaluated, medium with added 0.8% CaCO3 showed good growth of LAB at dilution 104 to 105. The higher the dilution of LAB were detected, the higher number of LAB population correspond to the samples. In this study, Tualang honey contained high numbers of LAB. The pH of honey samples varies from 3.5 to 5.7 with Tualang honey showing the lowest pH value of 3.5 (Table 1). A total of twenty-five isolates that showed clear zones on MRS agar with 0.8% CaCO3 and catalase negative were gram-positive stained and results showed that the LAB isolates were 52% rod-shaped and 48% coccus-shaped. Four of these LAB isolates namely, HS, HC, HH, and HM were selected for antifungal study against five strains of pathogenic Candida spp.
Table 1. Characterization of LAB that showed high antifungal activity against Candida species.
LAB, lactic acid bacteria; MRS, de Man, Rogosa & Sharpe; HS, Lactobacillus plantarum; HH, L. curvatus; HC, Pediococcus acidilactici; HM, P. pentosaceus.
Identification of LAB isolates by API 50 CHL and 16S rDNA
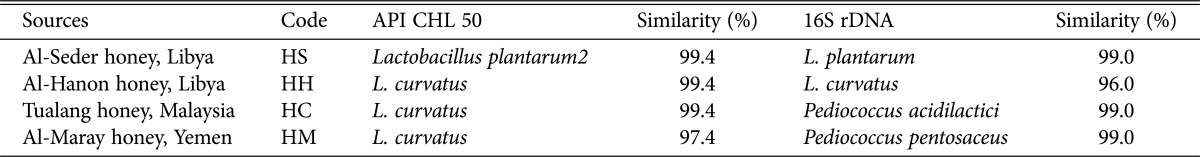
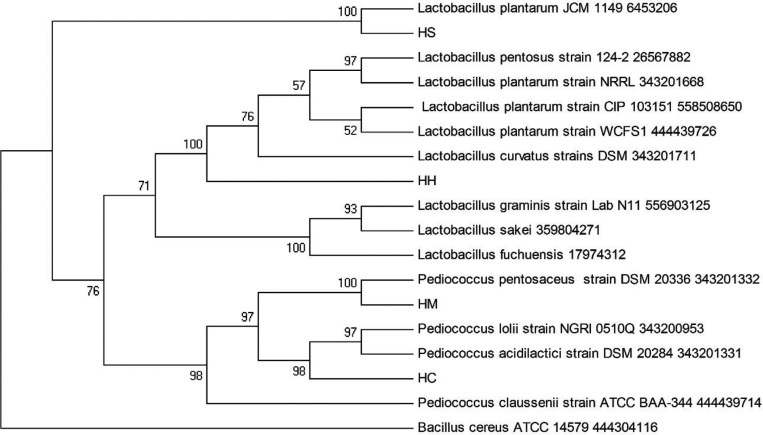
The identification of four LAB isolates from honey samples that showed antifungal activity against five strains of pathogenic Candida spp. is presented in Table 2. The results from API 50 CHL kit identified the isolate HS from Al-Seder honey, as Lactobacillus plantarum2, and other three isolates are HH from Al-Hanon honey, HC from Tualang honey, and HM from Al-Maray as L. curvatus. The results from 16S rDNA gene sequence identified HS as L. plantarum, HH as L. curvatus, HC as Pediococcus acidilactici, and HM as P. pentosaceus. Phenotypic and 16S rDNA molecular characterization of these isolates showed high degree of similarity of Lactobacillus and Pediococcus which belong to LAB. In this study, the sequencing of 16S rDNA gene of all isolates was confirmed by phylogenetic analysis. The phylogenetic tree was constructed by the neighbour-joining method as shown in Fig. 1, the isolate HS was the most closely related to the species L. plantarum 645320660 and isolate HH was the most closely related to the species L. curvatus 343201711 (Fig. 1) with similarities 99% and 96% in the 16S rDNA gene sequences, respectively. While isolate HC was the most closely related to the species Pediococcus acidilactici 343201331, supporting the 100% value from bootstrap analysis of the phylogenetic tree (Fig. 1) with 99% similarity in their 16S rDNA gene sequences. The isolate HM was closely related to the species Pediococcus pentosaceus 343201332 with 99% similarity in the 16S rDNA gene sequences.
Table 2. Similarity index of LAB isolated from honey samples as determined by API 50CHL and 16S rDNA.
LAB, lactic acid bacteria.
Fig. 1. Phylogenetic tree of partial 16S rDNA sequences of isolated strains and sequences of identified bacteria in the nucleotide database of GenBank. The bar indicates 1% sequence divergence.
Sensitivity of Candida spp. to antifungal agents
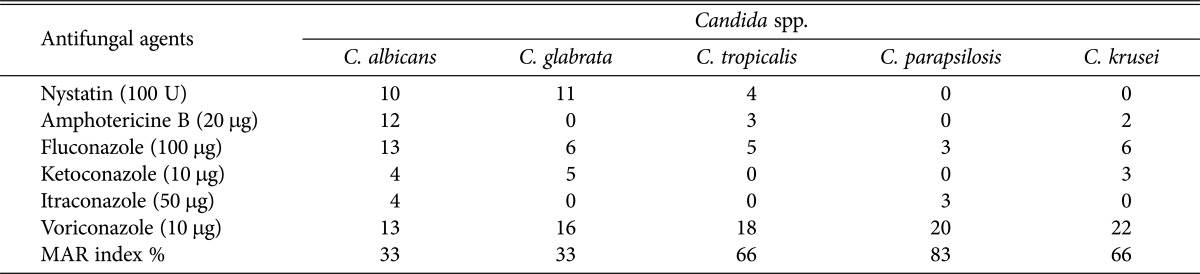
The sensitivity of Candida spp. to antifungal agents varied with species and antifungal agents evaluated with the diameter of inhibition zone varied between 0 and 22 mm. All the Candida spp. were sensitive to voriconazole (10 µg), but C. tropicalis ATCC750 and C. parapsilosis ATCC22019 were resistant to ketoconazole (10 µg) and C. glabrata ATCC2001 and C. tropicalis ATCC750 were resistant itraconazole (50 µg). It was observed that C. albicans ATCC14053 was sensitive to nystatin (100 U), amphotericine B (20 µg), and fluconazole (100 µg) while C. tropicalis ATCC750, C. parapsilosis ATCC22019, and C. krusei ATCC6258 were resistant to nystatin, amphotericine B and fluconazole. It was also observed that C. glabrata was sensitive to nystatin but very resistant to amphotericine B and itraconazole. The MAR index was between 33 and 83. The highest MAR index percentage (83%) was noted for C. parapsilosis compared to 33% exhibited by both C. albicans and C. glabrata, and 66% showed by both C. tropicalis and C. krusei (Table 3).
Table 3. Susceptibility of Candida spp. to antifungal drugs measured by diameter of inhibition (mm) zone around the discsa.
MAR, multiple antifungal resistant.
aDiameter of inhibition zone around the discs (mm). Diameter of paper discs = 6 mm.
Antifungal activity of LAB isolates against Candida spp. by the dual agar overlay method
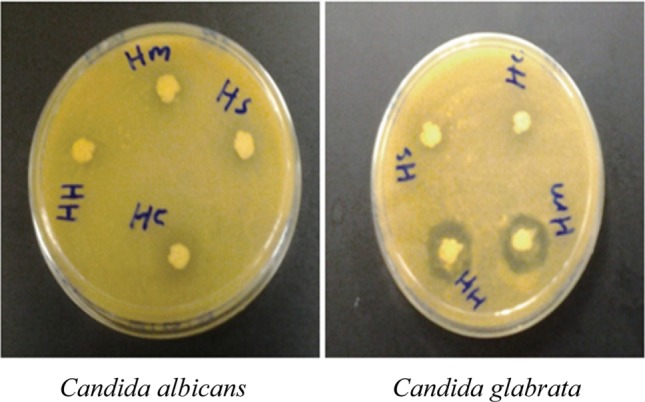
Growth of all Candida spp. were inhibited by all four LAB isolated from different honey samples, by the dual agar overlay method, especially the growth of C. glabrata ATCC2001, where this yeast was strongly inhibited by P. pentosaceus and L. curvatus isolated from Al-Maray honey, Yemen (Sample HM) and Al-Hanon honey, Libya (Sample HH), respectively. While growth of C. albicans ATCC14053 and C. tropicalis ATCC750 were moderately inhibited by all LAB isolates with inhibitory zone (6~10mm) (Table 4, Fig. 2).
Table 4. Growth inhibition zone of Candida species by LAB isolated from honey by dual agar overlay method after 24 hr incubation at 30℃a.
LAB, lactic acid bacteria; HS, Lactobacillus plantarum; HH, L. curvatus; HC, Pediococcus acidilactici; HM, P. pentosaceus.
aInhibitory activity of selected lactic acid bacteria isolates against Candida spp. after 24-hr incubation at 30℃ by dual agar overlay method. The inhibition was measured using the following scales: (−), no inhibition; (+), inhibition zone of less than 6mm; (++), inhibition zone of 6~10 mm; (+++), inhibition zone of 10~15 mm; (++++), inhibition zone of more than 15 mm.
Fig. 2. Growth inhibition of the isolates against Candida spp. by overlay method after 24 hr incubation at 30℃. HS, Lactobacillus plantarum; HH, L. curvatus; HC, Pediococcus acidilactici; HM, P. pentosaceus.
Antifungal activity of LAB supernatant against Candida spp. by well diffusion method
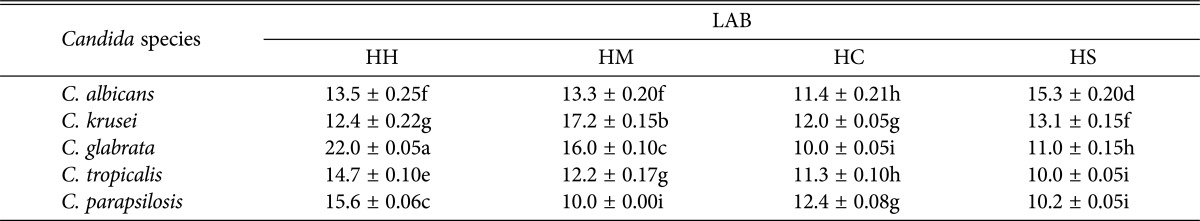
Four LAB isolates (HS, HC, HH, and HM) were selected for further evaluation on the antifungal activity of CFS against five strains of pathogenic Candida spp. using the agar well diffusion method. It was observed that all the CFS of LAB had significantly (p < 0.05) inhibited the growth of the pathogenic Candida spp. with the inhibition diameter between 10.0 and 17.2 mm while the greatest inhibition of 22.0 mm was exceptionally exhibited by HH against C. glabrata ATCC2001 (Table 5). The CFS of L. curvatus in the sample HH had significantly (p < 0.05) inhibited the growth of C. glabrata ATCC2001, C. parapsilosis ATCC2201, and C. tropicalis ATCC750 with inhibitory zones 22.0, 15.6, and 14.7mm, respectively. In addition, CFS of P. pentosaceus in the sample HM was significantly (p < 0.05) effective against C. krusei, C. glabrata, and C. albicans evident by inhibition zones of 17.2, 16.0, and 13.3 mm, respectively. Subsequently, CFS of L. plantarum in the sample HS had significantly (p < 0.05) inhibited the growth of C. albicans and C. krusei with inhibition zone 15.3 and 13.1 mm, respectively.
Table 5. Inhibition zone of Candida species by CFS using agar well diffusion method after 24-hr incubation at 37℃a.
The results are expressed as mean ± standard deviations of values obtained from triplicate experiments. Means with different letters were significantly different (p < 0.05).
CFS, cell free supernatant; LAB, lactic acid bacteria; HH, Lactobacillus curvatus; HM, Pediococcus pentosaceus; HC, P. acidilactici; HS, L. plantarum.
aDiameter of growth inhibitory zone was measured in millimeter after 24 hr, size the wells was 6 mm.
DISCUSSION
The presence of LAB in honey was reported by several researchers [30,31,32,33]. Aween et al. [34] isolated LAB from honey and the isolates were identified as strains of L. acidophilus that have antibacterial activities against gram-positive and gram-negative bacteria. In this study, LAB was detected in 10 from the 15 honey samples with variable antifungal activity against Candida spp. Four of the LAB that displayed good antifungal activities against Candida spp. were identified as L. plantarum HS, P. acidilactici HC, L. curvatus HH, and P. pentosaceus HM. Atanassova et al. [35], reported that L. paracasei subsp. paracasei M3 had antifungal activity against C. albicans, C. pseudointermedia, and C. blankii. Similarly, Jin et al. [36] also observed that strains of Pendiococcus sp. had strong antifungal activity against C. albicans ATCC10231 and C. parapsilosis ATCC22019 but moderate activity against C. tropicalis ATCC13803 and C. kefir ATCC46764. Ogunshe et al. [37] reported that L. acidophilus and L. plantarum from vaginal had antifungal activity against strains of pathogenic Candida spp. Cizeikiene et al. [38] also found that Pediococcus acidilactici KTU05-7, Pediococcus pentosaceus KTU05-8, KTU05-9 and KTU05-10 isolated from food had inhibitory activity against C. parapsilosis including Fusarium culmorum, Penicillium chrysogenum, Aspergillus fumigatus, Aspergillus versicolor, Penicillium expansum, Aspergillus niger, and Debaryomyces hansenii. In this study the L. plantarum isolated from Seder honey, Libya showed antifungal activity against Candida spp. especially C. albicans. Similarly, Adeniyi and Iveren [39] reported that the CFS produced by L. plantarum isolated from fresh salad vegetables had higher antifungal activity against C. albicans ATCC90029 with inhibition zone (25 mm). Oluwafemi and Adetunji [40] reported that L. plantarum isolated from Oqi had inhibitory activity against C. albicans. Similarly, the antifungal activity of L. plantarum were generally reported by several researchers against different fungi. Laref and Guessas [41] reported that five strains of L. plantarum isolated from silage, camel milk, and carrot had antifungal activity against Aspergillus spp., Fusarium roseum, Trichoderma spp., Penicilium spp., and Stemphylium spp. In this study, the highest zone of inhibition (> 15 mm and 10~15 mm) was recorded by P. pentosaceus HM and L. curvatus HH, respectively against C. glabrata ATCC2001. The strong antifungal activity by P. pentosaceus from foods was also supported by Muhialdin et al. [42] where they observed that L. pentosus G004, L. fermentum Te007, and P. pentosaceus Te010 isolated from Malaysian fermented foods and fruits had strong antifungal activity against Aspergillus oryzae. Their study also observed that LAB isolated from honey samples had good antifungal activity against Candida spp. as evaluated by the dual agar overlay method. In addition, it was also established that CFS of LAB show good inhibitory activity when evaluated by the well diffusion method. In this study, the highest antifungal activity was obtained with CFS of L. curvatus HH that showed significant (p < 0.05) antifungal activity against C. glabrata ATCC2001 with inhibition zone of 22.0 mm.
Candida spp. are not easily killed by normal antifungal agents used for health therapy. Candida spp. used in this study were resistant to several antifungal agents such as amphotericin B, fluconazole, and itraconazole except for voriconazole and fluconazole. Voriconazole is highly active against all Candida spp. while fluconazole is moderately effective against C. albicans. This is in agreement with Al-Abeid et al. [43] who reported that non-albicans spp. showed higher resistance rates against fluconazole than C. albicans. Our findings depicted that both the LAB cells and their CFS isolated from honey samples could inhibit the growth of Candida spp. and similar results were obtained by Lertcanawanichakul [44] who reported that the supernatant produced by Lactococus lactis showed inhibitory activity against C. albicans DMST 5239. The results from this study are in agreement with previous studies of Verdenelli et al. [45] who reported that L. rhamnosus and L. paracasei isolated from human stool had antifungal activity against C. albicans ATCC 10291, while Kariptaş et al. [46], observed that Lactobacillus isolated from human stool had antifungal activity against C. albicans (M29, M36), C. parapsilosis (M25, M26), C. famata (M28), and C. guilliermondii (M38) isolated from blood cultures. Recently, Chew et al. [47] reported that the CFS produced by the probiotic L. rhamnosus GR-1 and L. reuteri RC-14 have high antagonistic activities against five strains of C. glabrata. This is consistent with Rönnqvist et al. [48] who also reported that CFS produced by L. fermentum Ess-1 isolated from human had strong antifungal activity against C. albicans and C. glabrata. Sungsri et al. [49] also report on L. paracasei inhibits the growth of C. albicans BCC6120 by using dual agar overly method. The antimicrobial properties of CFS is contributed by the metabolites produced by LAB as reported by Olofsson et al. [50]. LAB produced organic acids, hydrogen peroxide, diacetyl, and bacteriocins are among others that have both antibacterial and antifungal activity. The current study shows that bacteria cells and their CFS isolated from different geographic location of natural honey samples have antifungal activity against Candida spp. It is no definite explanation on the mechanism of antifungal action of the CFS against Candida spp. due to the complex interactions between different metabolite compounds present in the CFS. Certain strains of LAB are able to produce natural antifungal compounds that can inhibit the growth of Candida spp. This may suggest that accumulation of soluble compounds in the CFS from LAB is responsible for inhibiting the growth of Candida spp.
The results obtained in this study indicated that LAB isolated from honey produced bioactive compounds that can be used to inhibit growth of the pathogenic Candida spp. namely, C. albicans ATCC14053, C. glabrata ATCC2001, C. tropicalis ATCC750, C. parapsilosis ATCC22019, and C. krusei ATCC6258, that often cause many human infections. The CFS of these LAB isolates showed greater inhibitory activity than the cells against Candida spp.
ACKNOWLEDGEMENTS
The authors would like to thank laboratory the staff at faculty of Science and Technology, Universiti Sains Islam Malaysia (USIM) and Universiti Kebangsaan Malaysia (UKM) for their assistance during the course of the study. I would also extend my gratitude to Dr. Mohmad Alshalmini from the Faculty of Agriculture, University Putra Malaysia and Ratuah Mohamed from the Faculty of Science and Technology, Universiti Kebangsaan Malaysia for their persistent encouragement and supports.
References
- 1.Weston RJ, Brocklebank LK. The oligosaccharide composition of some New Zealand honeys. Food Chem. 1999;64:33–37. [Google Scholar]
- 2.Molan P. The antibacterial properties of honey. Chem N Z. 1995;59:10–14. [Google Scholar]
- 3.Endo A, Futagawa-Endo Y, Dicks LM. Isolation and characterization of fructophilic lactic acid bacteria from fructose-rich niches. Syst Appl Microbiol. 2009;32:593–600. doi: 10.1016/j.syapm.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Adeniyi BA, Ayeni FA, Ogunbanwo ST. Antagonistic activities of lactic acid bacteria isolated from Nigerian fermented dairy food against organisms implicated in urinary tract infection. Biotechnology. 2006;5:183–188. [Google Scholar]
- 5.Sezer Ç, Güven A. Investigation of bacteriocin production capability of lactic acid bacteria isolated from foods. Kafkas Univ Vet Fak Derg. 2009;15:45–50. [Google Scholar]
- 6.Šuškovic J, Kos B, Beganović J, Leboš Pavunc A, Habjanič K, Matošić S. Antimicrobial activity: the most important property of probiotic and starter lactic acid bacteria. Food Technol Biotechnol. 2010;48:296–307. [Google Scholar]
- 7.Carlson P, Richardson M, Paavonen J. Evaluation of the Oricult-N dipslide for laboratory diagnosis of vaginal candidiasis. J Clin Microbiol. 2000;38:1063–1065. doi: 10.1128/jcm.38.3.1063-1065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 10.Ramage G, Martínez JP, López-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 11.Mishra NN, Prasad T, Sharma N, Payasi A, Prasad R, Gupta DK, Singh R. Pathogenicity and drug resistance in Candida albicans and other yeast species: a review. Acta Microbiol Immunol Hung. 2007;54:201–235. doi: 10.1556/AMicr.54.2007.3.1. [DOI] [PubMed] [Google Scholar]
- 12.Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58(Suppl 2):2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 13.Pikman R, Ben-Ami R. Immune modulators as adjuncts for the prevention and treatment of invasive fungal infections. Immunotherapy. 2012;4:1869–1882. doi: 10.2217/imt.12.127. [DOI] [PubMed] [Google Scholar]
- 14.Girmenia C, Menichetti F. Antimicrobial prophylaxis in hematology. In: Maschmeyer G, Rolston KV, editors. Infections in hematology. Berlin: Springer; 2015. pp. 275–296. [Google Scholar]
- 15.Kaškoniené V, Venskutonis PR. Floral markers in honey of various botanical and geographic origins: a review. Compr Rev Food Sci Food Saf. 2010;9:620–634. doi: 10.1111/j.1541-4337.2010.00130.x. [DOI] [PubMed] [Google Scholar]
- 16.Moussa A, Saad A, Djebli ND, Meslem A, Benhalima AE. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Int J Microbiol Res. 2011;2:276–279. doi: 10.1016/S2221-1691(12)60096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omafuvbe BO, Akanbi OO. Microbiological and physicochemical properties of some commercial Nigerian honey. Afr J Microbiol Res. 2009;3:891–896. [Google Scholar]
- 18.Koc AN, Silici S, Ercal BD, Kasap F, Hörmet-Öz HT, Mavus-Buldu H. Antifungal activity of Turkish honey against Candida spp. and Trichosporon spp: an in vitro evaluation. Med Mycol. 2009;47:707–712. doi: 10.3109/13693780802572554. [DOI] [PubMed] [Google Scholar]
- 19.Bulgasem BY, Hassan Z, Abdalsadiq NK, Yousoff WM, Musa EM, Lani MN. Anti-adhesion activity of lactic acid bacteria supernatant against human pathogenic Candida species biofilm. Health Sci J. 2015;9:3. [Google Scholar]
- 20.De Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of Lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 21.Panthavee W, Saithong P, Worawuthiyana N. Identification and evaluation of lactic acid bacteria for Pla-som (fermented fish) starter; The 2nd International Conference on Fermentation Technology for Value Added Agricultural Products; 2007 May 23-25; Khon Kaen, Thailand. [Google Scholar]
- 22.Ogunbanwo ST. Functional properties of lactic acid bacteria isolated from Ogi and Fufu, two Nigerian fermented foods. Adv Food Sci. 2005;27:14–21. [Google Scholar]
- 23.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 24.Subramani S, Vignesh S. MAR index study and MDR character analysis of a few golden staph isolates. Asian J Pharm Life Sci. 2012;2:151–154. [Google Scholar]
- 25.Magnusson J, Schnürer J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl Environ Microbiol. 2001;67:1–5. doi: 10.1128/AEM.67.1.1-5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamminen M, Joutsjoki T, Sjöblom M, Joutsen M, Palva A, Ryhänen EL, Joutsjoki V. Screening of lactic acid bacteria from fermented vegetables by carbohydrate profiling and PCR-ELISA. Lett Appl Microbiol. 2004;39:439–444. doi: 10.1111/j.1472-765X.2004.01607.x. [DOI] [PubMed] [Google Scholar]
- 27.Magnusson J, Ström K, Roos S, Sjögren J, Schnürer J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol Lett. 2003;219:129–135. doi: 10.1016/S0378-1097(02)01207-7. [DOI] [PubMed] [Google Scholar]
- 28.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Argueso T, Rodriguez-Navarro A. Microbiology of ripening honey. Appl Microbiol. 1975;30:893–896. doi: 10.1128/am.30.6.893-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahiru B, Mehari T, Ashenafi M. Yeast and lactic acid flora of tej, an indigenous Ethiopian honey wine: variations within and between production units. Food Microbiol. 2006;23:277–282. doi: 10.1016/j.fm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Hosny I, El-Ghani SA, Nadir AS. Nutrient composition and microbiological quality of three unifloral honeys with emphasis on processing of honey probiotic youghurt. Glob Vet. 2009;3:107–112. [Google Scholar]
- 33.Forsgren E, Olofsson TC, Váasquez A, Fries I. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie. 2010;41:99–108. [Google Scholar]
- 34.Aween MM, Hassan Z, Muhialdin BJ, Eljamel YA, Al-Mabrok AS, Lani MN. Antibacterial activity of Lactobacillus acidophilus strains isolated from honey marketed in Malaysia against selected multiple antibiotic resistant (MAR) gram-positive bacteria. J Food Sci. 2012;77:M364–M371. doi: 10.1111/j.1750-3841.2012.02776.x. [DOI] [PubMed] [Google Scholar]
- 35.Atanassova M, Choiset Y, Dalgalarrondo M, Chobert JM, Dousset X, Ivanova I, Haertlé T. Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int J Food Microbiol. 2003;87:63–73. doi: 10.1016/s0168-1605(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 36.Jin L, Tao L, Pavlova SI, So JS, Kiwanuka N, Namukwaya Z, Saberbein BA, Wawer M. Species diversity and relative abundance of vaginal lactic acid bacteria from women in Uganda and Korea. J Appl Microbiol. 2007;102:1107–1115. doi: 10.1111/j.1365-2672.2006.03147.x. [DOI] [PubMed] [Google Scholar]
- 37.Ogunshe AA, Omotoso MA, Bello VB. The in vitro antimicrobial activities of metabolites from Lactobacillus strains on Candida species implicated in Candida vaginitis. Malays J Med Sci. 2011;18:13–25. [PMC free article] [PubMed] [Google Scholar]
- 38.Cizeikiene D, Juodeikiene G, Paskevicius A, Bartkiene E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control. 2013;31:539–545. [Google Scholar]
- 39.Adeniyi B, Iveren D. Antifungal capacity of lactic acid bacteria isolated from salad vegetables. Afr J Biomed Res. 2011;14:137–141. [Google Scholar]
- 40.Oluwafemi F, Adetunji AF. Antimicrobial activities of lactic acid bacteria isolated from traditionally-fermented maize (ogi) against Candida albicans. J Appl Biosci. 2011;41:2820–2835. [Google Scholar]
- 41.Laref N, Guessas B. Antifungal activity of newly isolates of lactic acid bacteria. Innov Rom Food Biotechnol. 2013;13:80–88. [Google Scholar]
- 42.Muhialdin BJ, Hassan Z, Sadon SK. Antifungal activity of Lactobacillus fermentum Te007, Pediococcus pentosaceus Te010, Lactobacillus pentosus G004, and L. paracasi D5 on selected foods. J Food Sci. 2011;76:M493–M499. doi: 10.1111/j.1750-3841.2011.02292.x. [DOI] [PubMed] [Google Scholar]
- 43.Al-Abeid HM, Abu-Elteen KH, Elkarmi AZ, Hamad MA. Isolation and characterization of Candida spp. in Jordanian cancer patients: prevalence, pathogenic determinants, and antifungal sensitivity. Jpn J Infect Dis. 2004;57:279–284. [PubMed] [Google Scholar]
- 44.Lertcanawanichakul M. Isolation and selection of anti-Candida albicans producing lactic acid bacteria. Walailak J Sci Technol. 2005;2:179–187. [Google Scholar]
- 45.Verdenelli MC, Ghelfi F, Silvi S, Orpianesi C, Cecchini C, Cresci A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur J Nutr. 2009;48:355–363. doi: 10.1007/s00394-009-0021-2. [DOI] [PubMed] [Google Scholar]
- 46.Kariptaş E, Tulumoğlu S, Erdem B. Antifungal effects of Lactobacillus spp. bacteria on Candida yeast. Kafkas Univ Vet Fak Derg. 2010;16:1061–1064. [Google Scholar]
- 47.Chew SY, Cheah YK, Seow HF, Sandai D, Than LT. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J Appl Microbiol. 2015;118:1180–1190. doi: 10.1111/jam.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rönnqvist D, Forsgren-Brusk U, Husmark U, Grahn-Håkansson E. Lactobacillus fermentum Ess-1 with unique growth inhibition of vulvo-vaginal candidiasis pathogens. J Med Microbiol. 2007;56(Pt 11):1500–1504. doi: 10.1099/jmm.0.47226-0. [DOI] [PubMed] [Google Scholar]
- 49.Sungsri T, Lertcanawanichakul M, Siwayaprahm P. Isolation and selection of anti-Candida albicans metabolites producing lactic acid bacteria from various sources. KKU Res J. 2012;17:630–638. [Google Scholar]
- 50.Olofsson TC, Alsterfjord M, Nilson B, Butler È, Vásquez A. Lactobacillus apinorum sp. nov., Lactobacillus mellifer sp. nov., Lactobacillus mellis sp. nov., Lactobacillus melliventris sp. nov., Lactobacillus kimbladii sp. nov., Lactobacillus helsingborgensis sp. nov. and Lactobacillus kullabergensis sp. nov., isolated from the honey stomach of the honeybee Apis mellifera. Int J Syst Evol Microbiol. 2014;64(Pt 9):3109–3119. doi: 10.1099/ijs.0.059600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]