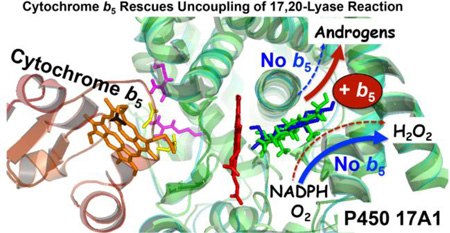
Abstract
Human cytochrome P450 17A1 is required for all androgen biosynthesis and is the target of abiraterone, a drug used widely to treat advanced prostate cancer. P450 17A1 catalyzes both 17-hydroxylation and subsequent 17,20-lyase reactions with pregnenolone, progesterone and allopregnanolone. The presence of cytochrome b5 (b5) markedly stimulates the 17,20-lyase reaction with little effect on 17-hydroxylation; however, the mechanism of this b5 effect is not known. We determined the influence of b5 on coupling efficiency—defined as the ratio of product formation to NADPH consumption—in a reconstituted system using these 3 pairs of substrates for the 2 reactions. Rates of NADPH consumption ranged from 4–13 nmol/min/nmol P450 with wild-type P450 17A1. For the 17-hydroxylase reaction, progesterone oxidation was the most tightly coupled (~50%) and negligibly changed upon addition of b5. Rates of NADPH consumption were similar for the 17-hydroxylase and corresponding 17,20-lyase reactions for each steroid series, and b5 only slightly increased NADPH consumption. For the 17,20-lyase reactions, b5 markedly increased product formation and coupling in parallel with all substrates, from 6% to 44% with the major substrate 17-hydroxypregnenolone. For the naturally occurring P450 17A1 mutations E305G and R347H, which impair 17,20-lyase activity, b5 failed to rescue the poor coupling with 17-hydroxypregnenolone (2–4%). When the conserved active-site threonine was mutated to alanine (T306A), both the activity and coupling were markedly decreased with all substrates. We conclude that b5 stimulation of the 17,20-lyase reaction primarily derives from more efficient use of NADPH for product formation rather than side products.
Keywords: cytochrome P450 17A1; 17,20-lyase; steroidogenesis; cytochrome b5; androgen
Graphical Abstract
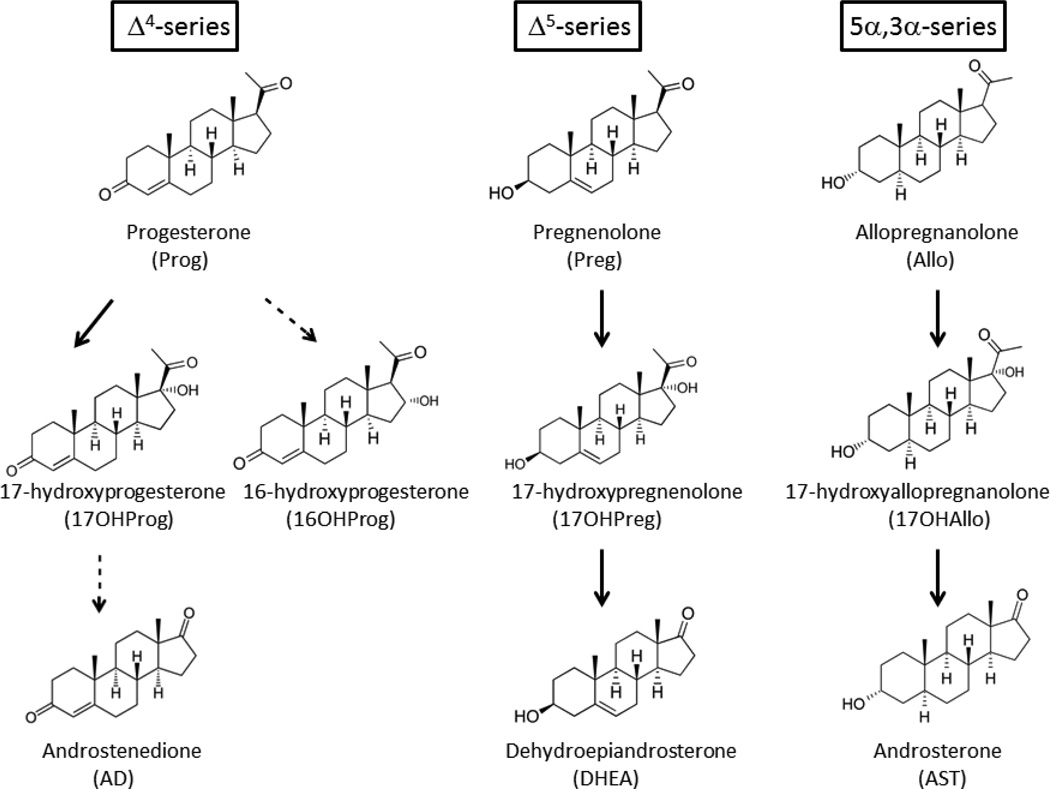
Human beings utilize 57 cytochrome P450 enzymes to perform a vast array of biological oxidation reactions. Of these, 6 enzymes are primarily expressed in the adrenals and/or gonads and catalyze all of the P450-mediated reactions necessary for steroid hormone biosynthesis1. The microsomal cytochrome P450 17A1 (steroid 17-hydroxylase/17,20-lyase, also called CYP17A1, P450c17) is essential for both cortisol and androgen production in these tissues. The 17-hydroxylase activity of human P450 17A1 demonstrates comparable activity with several substrates, including pregnenolone (pregn-5-en-3β-ol-20-one, Preg, Δ5-series), progesterone (pregn-4-ene-3,20-dione, Prog, Δ4-series), and allopregnanolone (5α-pregnan-3α-ol-20-one, Allo, 5α,3α-series)2,3. With Prog as the substrate, 20–25% of the product is 16α-hydroxyprogesterone4 (16OHProg, Figure 1). In contrast, its 17,20-lyase activity varies markedly with substrate, showing poor conversion of 17-hydroxyprogesterone (17OHProg) to androstenedione (AD), good conversion of 17-hydroxypregnenolone (17OHPreg) to dehydroepiandrosterone (DHEA), and even more efficient conversion of 5α-pregnane-3α,17α-diol-20-one (17OHAllo) to androsterone (AST, 5α-androstan-3α-ol-17-one)2,3. In addition, naturally occurring mutations have been identified in a conserved, active-site acidic residue (E305G)5 or the redox partner-binding site (R347H, R347C, R358Q)6,7, which preferentially impair 17,20-lyase activity.
Figure 1.
Major pathways of human steroidogenesis involving P450 17A1. The Δ4-series involves conversion of Prog to 17OHProg and 16OHProg, with a small amount of 17OHProg metabolized to AD; the Δ5-series, which is the dominant pathway to androgens in most circumstances, involves metabolism of Preg to 17OHPreg and then to DHEA; and the 5α,3α-series Allo to 17OHAllo to AST, an alternate pathway to dihydrotestosterone known as the “backdoor pathway.” Minor pathways are designated with dashed arrows.
Because P450 17A1 is responsible for all androgen production, it is an important target for the treatment of castration-resistant prostate cancer. Abiraterone acetate is in use, while galeterone, VT-464, and other compounds are being tested in clinical trials8. Inhibition of the 17,20-lyase reaction with abiraterone acetate treatment reduces circulating testosterone concentration beyond that achieved with medical castration therapy alone and prolongs survival in relapsed patients during androgen deprivation therapy9,10. Simultaneous inhibition of the 17-hydroxylase activity, however, causes hypertension and potassium loss due to the accumulation of the precursor steroid 11-deoxycorticosterone, a mineralocorticoid upstream of the enzymatic block11. For this reason, abiraterone acetate is administered with a synthetic glucocorticoid such as prednisolone to prevent these predictable side effects12,13. Current pharmaceutical interest in P450 17A1 is focused on the development of a selective inhibitor of 17,20-lyase activity, which would improve the safety profile and simplify therapy.
Both 17-hydroxylase and 17,20-lyase activities derive from a single active site with a limited steroid-binding pocket; consequently, the design of an active site-directed inhibitor targeting only the 17,20-lyase activity is challenging. The x-ray crystal structures of human P450 17A114,15 and of zebrafish P450s 17A1 and 17A216 have revealed subtle differences in substrate binding, which might account for differences in 17,20-lyase activities with 17-hydroxysteroid substrates; however, this structural information has not enabled the development of an active site-directed ligand that selectively inhibits 17,20-lyase activity. An alternative approach is to target allosteric sites on the enzyme surface that preferentially influence 17,20-lyase activity. One clue to advancing this strategy is the action of cytochrome b5 (b5), which selectively stimulates the 17,20-lyase activity17. This allosteric effect derives primarily from interactions between R347 and R358 of P450 17A1 with b5 residues E48, E49, and other adjacent residues on the two proteins18,19. The binding of b5 to P450 17A1 also alters the dynamics of residues distant from the sites of interaction and adjacent to the steroid-binding pocket20.
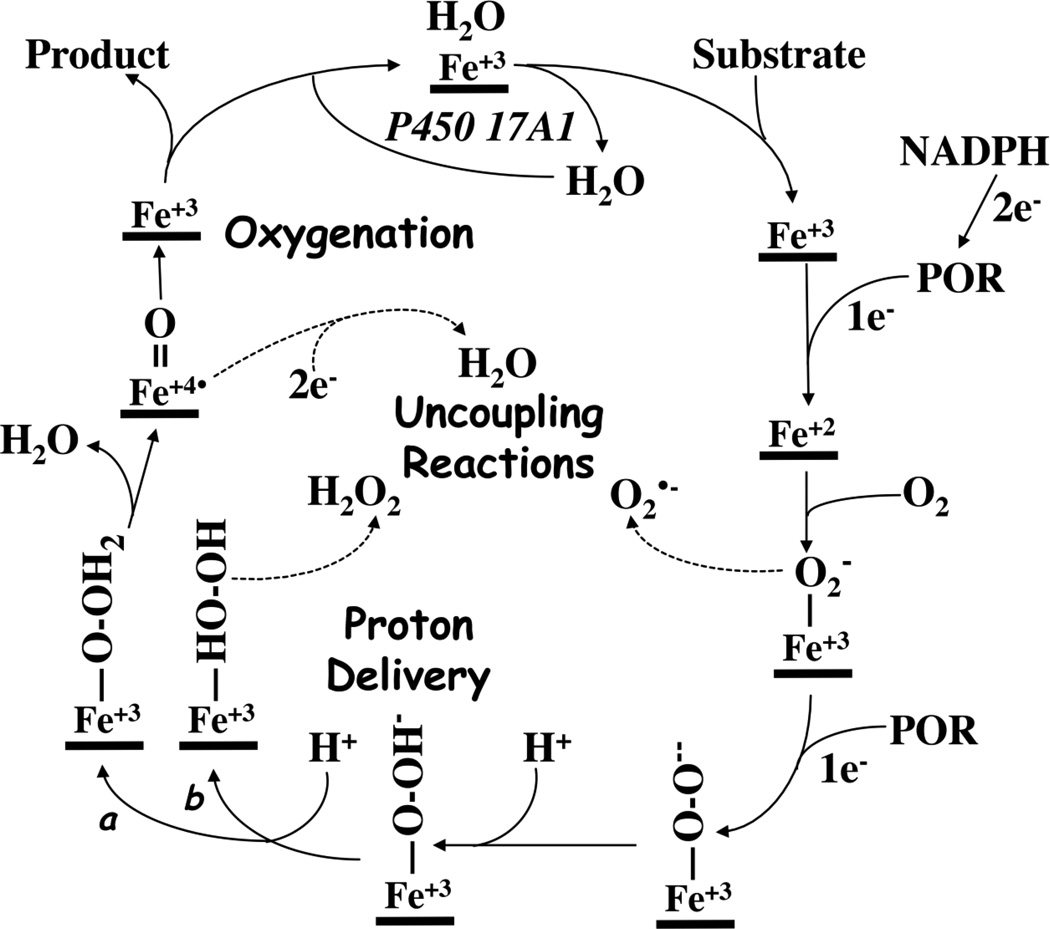
Our limited understanding of how b5 influences microscopic steps in the P450 17A1 catalytic cycle mutual to both 17-hydroxylase and 17,20-lyase activities (Figure 2) constitutes a significant impediment to exploiting the effect of b5 on the 17,20-lyase activity for the development of selective 17,20-lyase inhibitors. One possible mechanism of b5 action is to increase the coupling efficiency of reduced nicotinamide adenine dinucleotide phosphate (NADPH) and oxygen consumption to product formation for the 17,20-lyase reaction. Uncoupling of reducing equivalents during P450 cycling produces reduced oxygen species, including superoxide and hydrogen peroxide, rather than oxygenated products in vitro; however, whether similar uncoupling chemistry occurs in vivo is not known. Brock and Waterman reported that addition of b5 during progesterone metabolism did not change the rate of hydrogen peroxide formation for either rat or human P450 17A121, but this study did not address coupling for the 17,20-lyase reaction. To directly address this possible mechanism of b5 action on P450 17A1, we comprehensively compared NADPH consumption and product formation with several substrates for the 17-hydroxylase and 17,20-lyase reactions in the presence and absence of human b5, using wild-type human P450 17A1 and informative mutations in a reconstituted assay system with human P450-oxidoreductase (POR).
Figure 2.
Cytochrome P450 reaction cycle. Uncoupling reactions occur after binding of molecular oxygen, yielding superoxide or, if the second proton is not delivered to the distal oxygen (pathway a), hydrogen peroxide (pathway b). If compound I undergoes 2-electron reduction, uncoupling occurs via the oxidase shunt.
Experimental Procedures
Materials
Steroid substrates, reagents, medium, and solvents were purchased from Sigma-Aldrich (St. Louis, MO), Steraloids (Newport, RI), ThermoFisher Scientific (Pittsburgh, PA), or as specified. Enzymes for DNA modification were purchased from New England Biolabs (Beverly, MA). Protein determinations used the Coomassie Plus Reagent (Pierce, Rockford, IL). To prepare phospholipid stock solutions, phosphatidyl ethanolamine was dissolved in ethanol, and the solvent was evaporated under a stream of nitrogen. The residue was resuspended in 50 mM potassium phosphate buffer (pH 7.4) to a concentration of 1 mg/mL, and the resultant lipid dispersion was sonicated until nearly clear. Phosphatidyl choline was sonicated in 100 mM phosphate buffer at 1 mg/mL for 1 min on ice and centrifuged at 14,000 rpm for 4 min to remove precipitate. Phosphatidyl inositol was dissolved in 100 mM phosphate buffer at 1 mg/mL and sonicated on ice until clear. Stock solutions were stored at 4°C.
Plasmids
The full-length human P450 17A1 cDNA was synthesized by GenScripts (Piscataway, NJ) after optimizing for codon usage, mRNA secondary structure, and various cis-elements in transcription and translation. The N-terminal modification MALLLAVF was used to increase expression, and a 6x-His-tag was added at the C-terminus. The synthetic cDNA was restriction digested with NcoI and HindIII and ligated into the pLW01 plasmid; the resulting pLW01-human P450 17A1 plasmid was used for expression. N-27-human P450-oxidoreductase (POR) in pET2222 was a generous gift from Professor Walter L. Miller (University of California, San Francisco, CA). Plasmid pLW01-b5 and its use in the expression purification of tetrahistidine-tagged human b5 were described previously23,24.
Protein Expression in Escherichia coli and Purification
Human POR lacking 27 N-terminal residues was expressed and purified using modifications to previously described methods22. E. coli strain C41(DE3) transformed with plasmid pET22b-N-27POR-G3H6 was grown at 37°C in terrific broth supplemented with 0.1 mg/L of riboflavin to an OD of 1.8, at which time 0.4 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG) and 2% ethanol were added to initiate expression. The cultures (4 × 1L) were further incubated for ~20 hours at 25°C with shaking at 140 rpm. All purification procedures were performed at 4°C with protection to minimize light exposure. After cell lysis with a French press in buffer A (sterile phosphate-buffered saline [PBS] containing 15% glycerol, 1 mM phenylmethylsulfonyl fluoride, and SigmaFAST protease inhibitors at twice the recommended concentration), the recombinant proteins were solubilized with 0.5% cholate/0.5% Triton X-100/0.2% 4-nonylphenyl poly(ethylene glycol) (NP40), and the supernatant was collected after 15 min of centrifugation at 70,000 × g. A solution of saturated ammonium sulfate (pH 7.4) was added slowly to the supernatant until reaching 38% saturation, to precipitate impurities and to reduce proteolysis during subsequent steps. After centrifugation for 15 min at 19,000 × g, the supernatant was applied to His-Select resin (~6 mL, Sigma), washed with 10 mM imidazole and 0.1% Triton X-100 in buffer A, and eluted with 250 mM imidazole and 0.05% Triton X-100 in buffer A. The fractions containing the greenish POR proteins were diluted with ¼ volume of 1 M potassium phosphate buffer, pH 7.4, and then loaded onto 25 mL of Octyl-Sepharose resin (Sigma) pre-equilibrated with buffer B (PBS containing 20% glycerol and 1.7 M ammonium sulfate) to remove proteolyzed N-27 POR lacking the membrane-attachment peptide, which eluted in the flow-through and wash fractions. The column was washed with 3 column volumes of buffer B, and intact N-27 POR was eluted with buffer C (1/10 PBS, 20% glycerol, and 0.5% Triton-X 100). Potassium ferricyanide was added to the protein preparation at a final concentration of 10 µM before the buffer was exchanged to 50 mM potassium phosphate, pH 7.4, containing 20% glycerol using a PD-10 column (GE Healthcare, 8.3 mL bed volume). The final protein preparation was characterized using SDS-PAGE and UV spectroscopy (A455/A380 ratio = 1.18; A280/A455 ratio < 9.6)25.
Plasmids pLW01-human P450 17A1, wild-type and mutations, were used to transform C41(DE3) cells. After approximately 16 h at 37°C on Luria-Bertani plates containing 100 µg/mL carbenicillin, 3 colonies were transferred to 100 mL terrific broth containing 0.24 mM carbenicillin and incubated for 16–17 h at 30°C and 200 rpm. When the OD reached 3.5–4 and pH was 6.9–7.1, 10 mL of the overnight pre-culture were transferred to 1 L pre-warmed terrific broth containing 0.24 mM carbenicillin and 0.5 mM δ-aminolevulinic acid at 30°C, and cells were grown at 23°C with shaking at 140 rpm. When the OD reached 4–5, the culture was cooled with shaking at 110 rpm for 30 min to 20°C. IPTG was added to a final concentration of 100 µM, and after incubation at 20°C and 110 rpm for 30 min, absolute ethanol was also added to a final concentration of 1% (v/v). The culture was shaken at 110 rpm for 96–110 h until the expression of P450 was optimized with minimal P420 present, based on spectra of whole cell suspensions treated with CO. Cells were harvested by centrifugation at 2,830 × g for 15 min at 4°C.
The cell pellet from 3 L of culture was resuspended in 200 mL sonication buffer containing 100 mM potassium phosphate, pH 7.4, 30% glycerol, 2% Brij (nonaethylene glycol monododecyl ether), 1 mg deoxyribonuclease, 40 mg lysozyme, 1 µM butylated hydroxytoluene (BHT), 100 µM progesterone, SigmaFAST protease inhibitor at double-strength, and 0.1 mM (2-carboxyethyl)phosphine HCl (TCEP). The cell suspension was sonicated with 30 s pulses on ice for 10–15 cycles with 5–10 min cooling between pulses until a homogeneous preparation was obtained. The P450 was solubilized by stirring with 0.5% (w/v) cholate and 0.5% (v/v) NP40 for approximately 2 h, followed by ultracentrifugation at 23,700 × g for 30 min at 4°C. All subsequent purification steps were carried out in a cold room at 4–6°C.
The supernatant was loaded to a Ni-NTA column (~35 mL), which was pre-equilibrated with 100 mM potassium phosphate buffer, pH 7.4 containing 30% glycerol, 0.3 M NaCl, 0.1 mM TCEP, 1 µM BHT, and 100 µM progesterone. The column was washed with approximately 3 column volumes of equilibration buffer containing 0.1% (v/v) NP40 and 10 mM histidine. P450 17A1 was eluted with 0.1 M potassium phosphate buffer, pH 7.4 containing 30% glycerol, 0.3 M NaCl, 0.1 mM TCEP, 50 µM progesterone, 100 mM histidine, and 0.05% (v/v) NP40. After elution from Ni-NTA column, the reddish brown solution was mixed 1:1 with equilibration buffer (200 mM potassium phosphate buffer, pH 7.4 containing 20% glycerol, 0.8 M NaCl, 0.1 mM TCEP, and 50 µM progesterone) and loaded to an Octyl-Sepharose column (~150 mL), which was pre-equilibrated with the same buffer. The column was washed with 3 column volumes of equilibration buffer and then eluted with 100 mM potassium phosphate buffer, pH 7.4 containing 20% glycerol, 50 µM progesterone, 0.1 mM TCEP, and 0.3% NP40. To remove detergent and progesterone, P450-containing fractions were pooled and diluted five-fold with 30% glycerol containing 0.1 mM TCEP and loaded onto a hydroxyapatite column (~20 mL), which was pre-equilibrated with 50 mM potassium phosphate buffer, pH 7.4 containing 30% glycerol and 0.1 mM TCEP. The column was washed with 5 column volumes of equilibration buffer containing 0.05% NP40, and protein was then eluted with a linear gradient of 0.05 to 1 M potassium phosphate buffer, pH 7.4 containing 20% glycerol, 0.1 mM TCEP, and 0.05% (v/v) NP40. To concentrate the protein, purified P450 17A1 was reloaded to the hydroxyapatite column (~10 mL) as above and eluted with 0.5 M potassium phosphate buffer, pH 7.4 containing 20% glycerol, 0.1 mM TCEP, and 0.05% (v/v) NP40. The protein was stored in aliquots at −80°C and characterized for purity, yield, and activity using SDS-PAGE, spectra of CO-reduced enzyme26, and activity assay, respectively. The purified P450 17A1 contained 0.08 mol residual progesterone/mol P450 as determined by mass spectrometry.
Reconstituted Activity Assays
P450 17A1 (100–200 pmol, final concentration 0.1 µM) was mixed with 1 molar equivalent of POR with or without 1 molar equivalent of b5 and then reconstituted with phospholipids (20 µM final concentration, a freshly prepared 73:25:2 mixture of phosphatidyl choline, phosphatidyl ethanolamine, and phosphatidyl inositol from stock solutions) for 30 min on ice. The mixture was set at room temperature for 5 min and diluted to 0.9 mL with pre-warmed 100 mM potassium phosphate buffer (pH 7.4) and substrate at 10–20 µM final concentration in a 1 mL cuvette at 37°C. After 2 min, NADPH was added to a final concentration of 0.3 mM, and absorption at 340 nm was continuously recorded for the next 15 min in a Cary 4000 spectrophotometer at 37°C. The reaction mixture was quenched by addition of 0.1 mL of 3 N HCl and stored at −20°C until the products were analyzed with mass spectrometry. All experiments were performed in duplicate at least twice. The background rates of NADPH consumption were determined from equivalent incubations lacking P450. The NADPH consumption rate for control incubations without P450 was <6 nmol/min/nmol POR.
Steroid quantitation by LC-MS/MS
A 5–10 µL aliquot of the incubation was mixed with deuterated steroid internal standards in 200 µL methanol, 210 µL water, and 25 µL 50% aqueous 2-propanol and shaken at 120 rpm for 5 min. The mixture was applied to a supported liquid extraction column (Novum, Phenomenex), and the steroids were eluted with 3 washings of 0.7 mL methyl-t-butyl ether (MTBE). For measurement of Δ4-steroids, the organic eluates were concentrated under nitrogen, resuspended in 50% aqueous methanol, and analyzed with an Agilent 6490 tandem mass spectrometer equipped with an Agilent 1290 HPLC and Kinetex 50 × 2.1 mm, 3 µm particle size biphenyl column (Phenomenex) using gradient elution of methanol/water with 0.2 mM ammonium fluoride as described27. For measurement of Δ5- and 5α,3α-steroids, which were derivatized as oximes, a separate 10 µL aliquot of the quenched reaction mixture was first extracted, dried, and then resuspended in 50 µL 1 M ammonium hydroxide and 100 µL 1 M hydroxylamine hydrochloride, which was incubated at 90 °C for 30 minutes and subsequently re-extracted and dried. Table 1 gives retention times and precursor/product ion pairs for the targeted steroids. Intra-assay coefficients of variability (CV) ranged from 2–11% and inter-assay CV ranged from 2–16%.
Table 1.
Steroids measured by LC-MS/MS
| Steroid | Source | Precursor/Product ions (m/za) |
RT (min) | Internal standard |
|---|---|---|---|---|
| Pregnenolone | Cerilliant | 332.1/86.2 | 9.8 | Preg-d4 |
| 17OHPreg | Cerilliant | 348.1/330.3 | 8 | Preg-d4 |
| DHEA | Steraloids | 304/253.2 | 8.6 | DHEA-d5 |
| Progesterone | Cerilliant | 315.2/97 | 11 | Prog-d9 |
| 17OHProg | Cerilliant | 331.2/97 | 8 | 17OHProg-d8 |
| 16OHProg | Steraloids | 331.2/97 | 7.6 | 17OHProg-d8 |
| AD | Cerilliant | 287/97 | 8.9 | AD-d5 |
| Allo | Steraloids | 334.5/86 | 10.3 | Preg-d4 |
| 17OHAllo | Steraloids | 332.2/314.2 | 9.8 | Preg-d4 |
| Androsterone | Steraloids | 306/255.2 | 9.6 | DHEA-d5 |
m/z, mass to charge ratio; RT, retention time; internal standards were from C/D/N Isotopes except DHEA-d5, from Sigma-Aldrich
Calculation of Coupling Efficiencies
The rates of NADPH oxidation were calculated from the linear portion of the absorption at 340 nm versus time curves using the extinction coefficient ɛ = 6220 M−1•cm−1 and subtracting the background rates (nmol NADPH/min/nmol P450). The rates of product formation for the 17,20-lyase reactions were calculated by dividing the amount of 19-carbon product (AD, DHEA, AST) formed from the corresponding 17-hydroxysteroid substrate (17OHProg, 17OHPreg, 17OHAllo, respectively, corrected for recovery) by the duration of the incubation (example: nmol DHEA/min/nmol P450). The rates of product formation for the 17-hydroxlyase reactions were calculated by dividing the amount of total products formed (corrected for recovery) from substrates Prog, Preg, and Allo, including initial hydroxysteroid products and 19-carbon steroids formed via a second turnover, by the duration of the incubations. For incubations with Prog, both 16OHProg and 17OHProg products were included in calculations. All rates were expressed as nmol products/min/nmol P450. Coupling efficiency is defined as the rate of total product formation divided by the rate of NADPH consumption (nmol products/nmol NADPH). To determine coupling efficiencies for the 17,20-lyase reactions, the rates of 19-carbon steroid formation from 17-hydroxysteroid substrates were directly divided by the rates of NADPH consumption from the same experiment (example: nmol DHEA/nmol NADPH). To determine coupling efficiencies for the 17-hydroxlyase reactions, the NADPH consumption attributed to the 17,20-lyase reaction, derived from a second turnover using the initial 17-hydroxysteroid product as the substrate, was first subtracted based on coupling efficiencies from incubations with 17-hydroxysteroid substrates under the same conditions. The rate of 17-hydroxy (and 16α-hydroxy) product formation divided by the remaining NADPH consumption rate equals the coupling efficiency for the 17- (and 16α-) hydroxylase reaction(s).
Results
Expression and purification of human P450 17A1 and POR
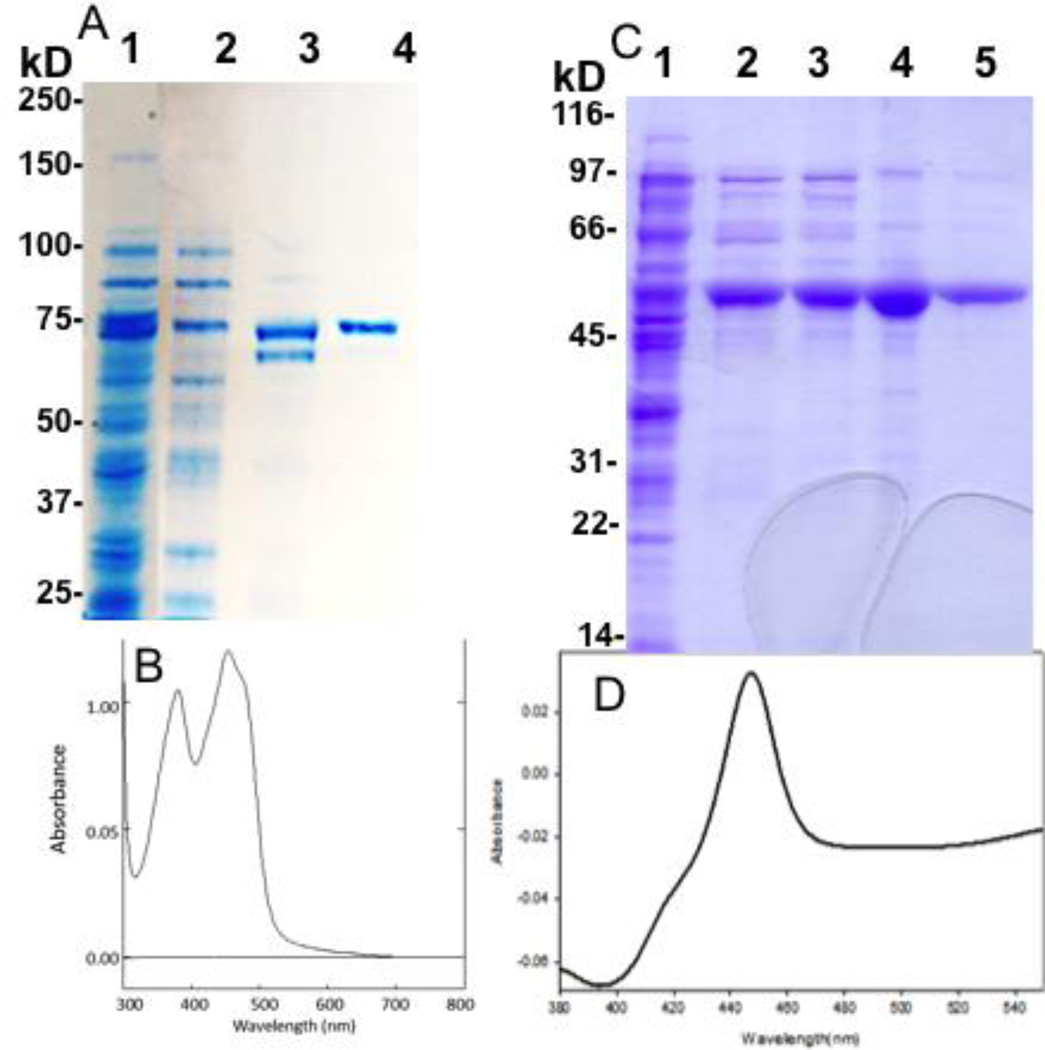
For experiments measuring NADPH consumption, only recombinant, purified proteins and lipids were used to minimize extraneous sources of activity and NADPH consumption. To mimic the in vivo enzyme system as closely as possible, we used only the human forms of P450 17A1, POR, and b5 and a phospholipid composition similar to that found in human adrenal microsomes28. A limitation to the use of human POR in recombinant assays is its vulnerability to proteolysis near the membrane-attachment site during purification despite strict precautions. Although we had found conditions (0.1 mg/L riboflavin, 0.4 mM IPTG, 2% ethanol) affording very high expression of functional protein, a significant amount of proteolysis occurred during affinity chromatography, yielding only ~50% uncleaved N-27 POR. An ammonium sulfate precipitation after solubilization to remove the responsible proteases increased the amount of uncleaved N-27 POR to about 80%. An Octyl-Sepharose column under high ionic strength following affinity chromatography efficiently removed the cleaved protein and gave a high recovery of purified N-27 POR, 110 nmol/L culture with >90% flavin content (Figure 3A, 3B).
Figure 3.
Purification of recombinant human POR and P450 17A1. Panel A, SDS-PAGE of N-27 POR-G3H6 preparation in crude lysate (lane 1), after ammonium sulfate precipitation (lane 2), after affinity chromatography (lane 3), and final preparation after Octyl-Sepharose (lane 4). Panel B, UV-visible spectrum of final N-27 POR preparation. Panel C, SDS-PAGE of P450 17A1-H6 preparation in crude solubilized lysate after ultracentrifugation (lane 1), after affinity chromatography (lane 2), after Octyl-Sepharose (lane 3), control P450 17A1 standard (lane 4), and final preparation after hydroxyapatite column (lane 5). Panel D, CO-reduced difference spectrum of purified P450 17A1 shows negligible P420.
For human P450 17A1, a codon-optimized vector yielded 900 nmol/L culture in the crude homogenate, and our purification strategy was optimized to primarily minimize P420 and obtain the best yield in a 2–3 day procedure. Consequently, low-temperature expression followed by sequential chromatography on Ni-NTA agarose, Octyl-Sepharose, and hydroxyapatite columns afforded 170 nmol/L culture of purified enzyme with 12% P420 (Figure 3C, 3D). Expression and purification of P450 17A1 mutations E305G, R347H, E305Q, and T306A followed similar procedures and gave smaller yields (80–110 nmol/L culture). Initial experiments compared reconstituted assays using full-length versus N-27 POR with P450 17A1 derived from the method of Wang et al29 versus the current codon-optimized plasmid. All combinations of POR and P450 17A1 preparations yielded equivalent activities (<5% difference), provided that the proteolytically cleaved POR species were removed with Octyl-Sepharose prior to reconstitution. Initial experiments also showed that activity plateaued at a POR:P450 ratio of 1:1, but calculated coupling efficiencies declined at higher POR:P450 ratios (not shown); consequently, a 1:1 ratio was used in all experiments.
Coupling efficiencies and effect of b5 with wild-type P450 17A1
For the 17-hydroxylase reaction in the absence of b5, the coupling efficiency was greatest for Prog at 46.0 ± 5.2% and lower for Preg and Allo (24.3 ± 2.3% and 9.1 ± 0.9%, respectively, Table 2). The rates of NADPH consumption were also highest for Prog (12 nmol product/min/nmol P450) and lowest for Allo. Addition of b5 to the incubations did not alter the coupling efficiency of Prog metabolism but increased the coupling efficiency for Preg and Allo to ~50%, similar to that of Prog metabolism without b5 (Table 2). Although we estimated and subtracted the NADPH consumption due to the subsequent metabolism of 17-hydroxysteroid intermediates from our calculations, which is significant for Preg and Allo metabolism, we cannot exclude some error in these measurements due to our deconvolution analysis.
Table 2.
NADPH consumption and product formation with wild-type P450 17A1
| Substrate | b5a | Product (min−1) b |
NADPH (min−1) b |
Coupling (%) |
b5 increase in coupling (fold) |
|---|---|---|---|---|---|
| Prog | − | 5.5 ± 0.8 | 12.0 ± 0.4 | 46.0 ± 5.2 | |
| Prog | + | 5.3 ± 0.2 | 13.0 ± 0.3 | 40.9 ± 1.8 | 0.9 |
| Preg | − | 1.7 ± 0.2 | 7.0 ± 0.3 | 24.3 ± 2.3 | |
| Preg | + | 2.6 ± 0.8 | 4.2 ± 0.6 | 61.3 ± 6.3 | 2.5 |
| Allo | − | 0.4 ± 0.0 | 4.8 ± 0.1 | 9.1 ± 0.9 | |
| Allo | + | 2.0 ± 0.1 | 4.3 ± 0.0 | 46.4 ± 2.0 | 5.1 |
| 17OHProg | − | 0.1 ± 0.0 | 12.1 ± 0.3 | 0.5 ± 0.2 | |
| 17OHProg | + | 1.1 ± 0.3 | 11.3 ± 0.1 | 10.2 ± 2.9 | 20.4 |
| 17OHPreg | − | 0.4 ± 0.0 | 6.9 ± 0.1 | 5.7 ± 0.1 | |
| 17OHPreg | + | 4.0 ± 0.3 | 9.2 ± 0.7 | 44.0 ± 0.5 | 7.7 |
| 17OHAllo | − | 0.7 ± 0.3 | 4.4 ± 0.3 | 15.5 ± 7.8 | |
| 17OHAllo | + | 3.9 ± 0.4 | 5.3 ± 0.1 | 73.9 ± 12.4 | 4.8 |
Molar ratios of P450:POR:b5=1:1:0 or 1:1:1;
Rates are nmol/min/nmol P450
For the 17,20-lyase reactions, the rates of NADPH consumption were similar to rates for the 17-hydroxylase reactions within each substrate series, and b5 increased NADPH consumption only 33% or less (Table 2). These results are in agreement with the effects of b5 on P450 2B4 catalysis, where stimulation of activity was a result of increased coupling of NADPH consumption to product formation30–33. Coupling efficiencies in the absence of b5, however, were much lower than for the corresponding 17-hydroxylase reactions with 17OHProg and 17OHPreg as the substrates. Addition of b5 increased the coupling efficiency for the 17,20-lyase reactions with 17OHProg from 0.5 ± 0.2% to 10.2 ± 2.9% and with 17OHPreg from 5.7 ± 0.1% to 44.0 ± 0.5%. Furthermore, these increases in coupling efficiency rather than changes in NADPH consumption accounted for essentially all of the increased rates of product formation upon addition of b5. In the 5α,3α-series, addition of b5 increased the coupling for 17OHAllo conversion to androsterone from 15.5 ± 7.8% to 73.9 ± 12.4%, which is the smallest increase in product formation for the 17,20-lyase reaction3 but the highest absolute value of coupling efficiency for any substrate in our study.
Coupling efficiencies and effect of b5 with mutations that are deficient in 17,20-lyase activity
Our data with wild-type b5 predict that P450 17A1 mutations that are selectively deficient of 17,20-lyase activity will show similar rates of NADPH consumption but low coupling efficiencies for the 17,20-lyase reactions and poor rescue upon addition of b5. For the 17-hydroxylase substrates Prog and Preg, mutation R347H6 displayed similar rates of NADPH consumption (74–108%) and product formation (70–118%) as wild-type P450 17A1, yielding coupling efficiencies within a factor of 0.65–1.3 that of wild-type enzyme (Table 3). For the 17,20-lyase substrates 17OHProg and 17OHPreg, NADPH consumption rates were also similar to wild-type P450 17A1; however, rates of product formation and coupling efficiencies were much lower than for the wild-type enzyme (Table 3, Figure 4). In the presence of b5, the coupling efficiencies for the 17,20-lyase reactions with 17OHProg and 17OHPreg as the substrates increased only to 0.8 ± 0.1% and 4.2 ± 1.8%, respectively. Thus, although differences in NADPH consumption rates are not markedly different from wild-type P450 17A1, the 17,20-lyase deficient mutation R347H shows poor coupling efficiencies for the 17,20-lyase reaction, which b5 cannot restore to normal.
Table 3.
NADPH consumption and product formation with mutations R347H and E305G
| Product | NADPH | Coupling | |||||
|---|---|---|---|---|---|---|---|
| Substrate | b5a | (min−1)b | %WT | (min−1)b | %WT | (%) | %WT |
| Mutation R347H | |||||||
| Prog | − | 4.2 ± 0.1 | 76 | 8.9 ± 0.3 | 74 | 47.6 ± 3.2 | 103 |
| Prog | + | 3.7 ± 0.1 | 70 | 14.0 ± 0.7 | 108 | 26.5 ± 2.3 | 65 |
| Preg | − | 2.0 ± 0.0 | 118 | 6.3 ± 0.4 | 90 | 31.4 ± 1.6 | 129 |
| Preg | + | 2.8 ± 0.1 | 108 | 4.4 ± 1.4 | 105 | 63.9 ± 20.8 | 104 |
| 17OHProg | − | 0.01 ± 0.00 | 10 | 7.5 ± 0.7 | 62 | 0.1 ± 0.0 | 20 |
| 17OHProg | + | 0.06 ± 0.01 | 5 | 7.4 ± 0.9 | 65 | 0.8 ± 0.1 | 8 |
| 17OHPreg | − | 0.1 ± 0.0 | 25 | 6.3 ± 0.4 | 91 | 2.1 ± 0.6 | 37 |
| 17OHPreg | + | 0.3 ± 0.2 | 8 | 7.2 ± 1.6 | 78 | 4.2 ± 1.8 | 10 |
| Mutation E305G | |||||||
| Prog | − | 1.3 ± 0.2 | 24 | 2.8 ± 0.2 | 23 | 48.3 ± 10.5 | 105 |
| Prog | + | 1.2 ± 0.3 | 23 | 6.1 ± 0.2 | 47 | 19.8 ± 3.6 | 48 |
| Preg | − | 0.3 ± 0.0 | 18 | 1.7 ± 0.2 | 24 | 19.4 ± 1.6 | 80 |
| Preg | + | 0.3 ± 0.0 | 12 | 1.9 ± 0.1 | 45 | 17.6 ± 1.9 | 29 |
| 17OHProg | − | 0.04 ± 0.00 | 35 | 5.5 ± 0.6 | 45 | 0.6 ± 0.1 | 120 |
| 17OHProg | + | 0.6 ± 0.1 | 55 | 7.2 ± 0.5 | 64 | 9.9 ± 0.7 | 97 |
| 17OHPreg | − | 0.04 ± 0.00 | 10 | 1.2 ± 0.1 | 17 | 3.2 ± 0.2 | 56 |
| 17OHPreg | + | 0.03 ± 0.01 | 1 | 2.1 ± 0.1 | 23 | 1.4 ± 0.0 | 3 |
Molar ratios of P450:POR:b5=1:1:0 or 1:1:1;
Rates are nmol/min/nmol P450
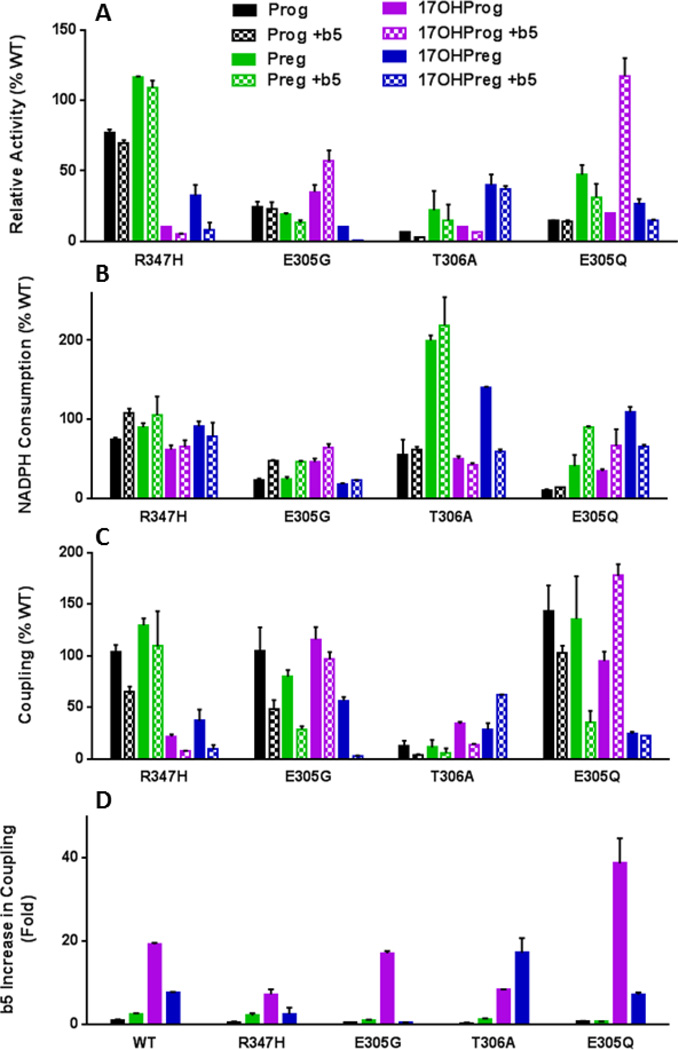
Figure 4.
Product formation rates (A), NADPH consumption rates (B), and coupling efficiencies (C) with various substrates for P450 17A1 mutations relative to wild-type enzyme, as well as b5 effects on coupling (D). Color code for each steroid matches text in Tables 2–4.
Mutation E305G was identified in a kindred with partial 17-hydroxylase deficiency and 17,20-lyase deficiency in the 17OHPreg pathway5,34. NADPH consumption and product formation rates with all substrates were consistently lower for mutation E305G than for wild-type P450 17A1, particularly for Preg and 17OHPreg substrates (Table 3, Figure 4). Similar to mutation R347H, the coupling efficiency for the conversion of 17OHPreg to DHEA was low and not increased upon addition of b5 (3.2 ± 0.2% and 1.4 ± 0.0%, respectively). In contrast, b5 increased the rate of product formation for the 17,20-lyase reaction with 17OHProg as the substrate as previously shown5, and the coupling efficiency was similar to wild-type P450 17A1 (Table 3). Consequently, the proportionately lower NADPH consumption and activity of the E305G mutant indicate that its lower activity in the absence of b5 is coupled to a similar extent as the wild-type enzyme (Figure 4).
Coupling efficiencies and effect of b5 on mutations in conserved residues known to affect formation of compound I in other P450s
Mutation T306A, analogous to mutation T252A in P450cam, exhibits both poor activity and coupling efficiency (<6%) with all substrates in the absence of b5 (Table 4). Curiously, b5 substantially increases the coupling efficiency for the 17,20-lyase reaction, particularly with 17OHPreg as the substrate, but not for the 17-hydroxylase reactions, as previously reported35. In contrast, mutation E305Q, analogous to mutation D251N in P450cam, demonstrates decreased catalytic properties similar to mutation E305G in the absence of b5, with generally reduced rates of NADPH consumption (10–109%) and product formation (15–47%) compared to wild-type enzyme and preferential 17,20-lyase impairment (Figure 4). The coupling of mutation E305Q is similar to that of the wild-type enzyme, and addition of b5 substantially increased product formation and coupling for the 17,20-lyase reaction over 30- and 6-fold with 17OHProg and 17OHPreg as the substrates, respectively (Table 4, Figure 4).
Table 4.
NADPH consumption and product formation with mutations T306A and E305Q
| Product | NADPH | Coupling | |||||
|---|---|---|---|---|---|---|---|
| Substrate | b5a | (min−1)b | %WT | (min−1)b | %WT | (%) | %WT |
| Mutation T306A | |||||||
| Prog | − | 0.4 ± 0.0 | 7 | 6.7 ± 2.2 | 56 | 6.0 ± 2.1 | 13 |
| Prog | + | 0.1 ± 0.0 | 2 | 8.1 ± 0.4 | 62 | 1.7 ± 0.1 | 4 |
| Preg | − | 0.4 ± 0.2 | 24 | 14.0 ± 0.4 | 200 | 2.7 ± 1.7 | 11 |
| Preg | + | 0.4 ± 0.3 | 15 | 8.9 ± 1.2 | 212 | 4.3 ± 2.8 | 7 |
| 17OHProg | − | 0.01 ± 0.00 | 10 | 6.0 ± 0.4 | 50 | 0.2 ± 0.0 | 40 |
| 17OHProg | + | 0.07 ± 0.01 | 6 | 4.8 ± 0.2 | 42 | 1.4 ± 0.1 | 14 |
| 17OHPreg | − | 0.2 ± 0.0 | 50 | 9.7 ± 0.1 | 140 | 1.7 ± 0.3 | 30 |
| 17OHPreg | + | 1.5 ± 0.1 | 38 | 5.4 ± 0.3 | 59 | 27.3 ± 0.3 | 62 |
| Mutation E305Q | |||||||
| Prog | − | 0.8 ± 0.0 | 15 | 1.2 ± 0.2 | 10 | 66.1 ± 11.3 | 144 |
| Prog | + | 0.7 ± 0.1 | 13 | 1.8 ± 0.0 | 14 | 41.9 ± 3.0 | 102 |
| Preg | − | 0.8 ± 0.1 | 47 | 3.0 ± 0.8 | 43 | 28.3 ± 10.0 | 116 |
| Preg | + | 0.8 ± 0.3 | 31 | 3.8 ± 0.1 | 90 | 21.2 ± 6.9 | 35 |
| 17OHProg | − | 0.02 ± 0.00 | 20 | 4.1 ± 0.3 | 34 | 0.5 ± 0.0 | 100 |
| 17OHProg | + | 1.3 ± 0.4 | 118 | 7.5 ± 2.4 | 66 | 18.1 ± 1.1 | 177 |
| 17OHPreg | − | 0.1 ± 0.0 | 25 | 7.5 ± 0.5 | 109 | 1.4 ± 0.1 | 25 |
| 17OHPreg | + | 0.6 ± 0.3 | 15 | 6.0 ± 0.2 | 65 | 9.8 ± 0.0 | 22 |
Molar ratios of P450:POR:b5=1:1:0 or 1:1:1;
Rates are nmol/min/nmol P450
Discussion
The major finding of this study is that most if not all of the b5-mediated increase in the rate of the P450 17A1-catalyzed 17,20-lyase reaction is quantitatively attributable to improvements in coupling efficiency, in agreement with the effects of b5 on catalysis by P450 2B430–33. These results are internally consistent with 3 series of substrates (Prog = Δ4-series, Preg = Δ5-series, and Allo = 5α,3α-series) and for 4 different mutations that impair 17,20-lyase activity (Figure 4). For wild-type P450 17A1, the rates of NADPH consumption are within a factor of 3.1 for all substrates tested (4.2–13.0 nmol/min/nmol P450), and addition of b5 changes these rates for each substrate <2-fold. In contrast, the turnover rates vary over 50-fold for these substrates in the absence of b5 (0.1–5.5 nmol/min/nmol P450), due to marked differences in coupling efficiencies (Table 2).
Our data are subject to the same limitations of all P450 studies using reconstituted assays with purified proteins. Changes in activity, particularly for the 17,20-lyase reaction, are observed depending on phospholipids used for reconstitution28. NADPH measurements required high concentrations of substrates and proteins, and results might otherwise vary with incubation conditions or using intact cells. The very poor turnover for some experiments, particularly with 17OHProg as the substrate, limits quantitative comparisons. Our methods might have under- or over-estimated the magnitude of corrections for sequential turnovers, and we did not account for minor products such as androst-5-ene-3β-ol formation in the presence of b5 with Preg as the substrate3. Nevertheless, the internal consistency of our results with different substrates and mutations that influence activity strengthen our conclusions.
The b5 residues E48 and E49 are critical for stimulation of 17,20-lyase activity18, and P450 17A1 mutations have been found in patients with the clinical syndrome of isolated 17,20-lyase deficiency at residues R347 and R3586, which impair interactions with redox partners POR and b536. Furthermore, cross-linking19 and electrochemistry37 experiments are consistent with a structural model in which R449, R347, and R358, of P450 17A1 form distributed electrostatic interactions with E42, E48, and E49 of b5, while hydrogen-bonding of K88 of P450 17A1 and E61 of b5 provides a second anchoring point for the heterodimeric complex19. Although the influence of b5 on 17,20-lyase activity has been conclusively demonstrated with biochemical2 and genetic38,39 evidence, the mechanism of how the interaction of these specific residues translates into a change in enzyme function has remained obscure. Our data presented herein provide a significant advance in our understanding of this mechanism.
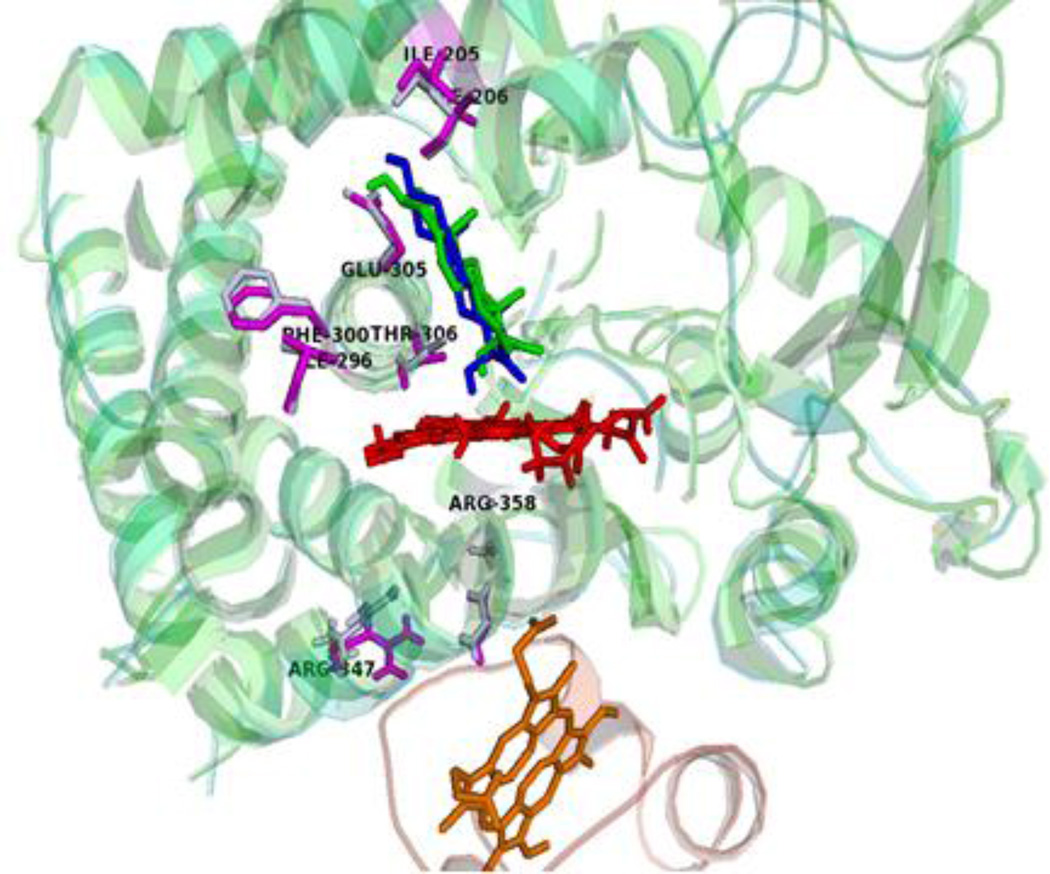
NMR studies with soluble forms of P450 17A1 and 15N-labeled b5 are consistent with direct interaction between the aforementioned side chains. Addition of wild-type P450 17A1 results in profound line broadening for resonances from b5 residues E48 and E49, but P450 17A1 mutations R347H, R358Q, and R449L do not produce these spectral changes20. In addition, NMR studies of abiraterone-bound P450 17A1 and b5 found disproportionate broadening of peaks derived from residues lining the active site upon mixing the two proteins, particularly I205 and F300 in the F- and I-helices, respectively40. The 15N-1H correlated resonances from I205, I206, I292, I296, and F300 undergo complex transitions during b5 titration, suggesting redistribution of conformational state populations, and for some of these residues, the peak features vary with both substrate and b5. Other unassigned resonances suspected to comprise the redox-partner binding site paradoxically sharpened rather than broadened. Thus, the binding of b5 to P450 17A1 induces alterations in the dynamics of residues both in the interaction surface and in those surrounding the active site in a substrate-specific manner20,40. Consequently, our data suggest that the binding of b5 to the P450 17A1•substrate complex alters the structure of the active site to facilitate formation of acyl carbon cleavage products, in agreement with a hypothesis put forward by Akhtar and coworkers41. Our data further demonstrate that these conformational changes reduce exposure to trajectories that allow for uncoupling reactions (Figure 5).
Figure 5.
Proposed mechanism for b5 stimulation of 17,20-lyase reaction. The structure of human P450 17A1 mutation A105L with 17OHPreg (PDB 4NKZ, green) superimposed on the structure of ternary complex (blue) formed with b5 (orange). The docking was performed using the programs HADDOCK with the distance constraints of two intermolecular cross-links previously identified19. The binding of b5 transmits structural changes to residues surrounding the active site (labeled and magenta) and modifies the orientation of 17OHPreg, which increases productive cycles and limits uncoupling reactions.
Our results are qualitatively similar to those reported using human P450 17A1 in nanodiscs with rat POR and rat b5 in that the 17,20-lyase reaction was very poorly coupled compared to the 17-hydroxylase reaction35. That study, however, did not comprehensively explore the effect of b5 on coupling efficiency, including the 5α,3α-substrates and the mutations deficient in 17,20-lyase activity. In addition, b5 did not restore the 17,20-lyase activity as robustly as observed in our experiments. The study of Khatri et al primarily addressed the mechanism of the 17,20-lyase reaction and the nature of the functional iron-oxygen species35, and the data were interpreted as favoring a peroxo-anion intermediate (Fe+3-OO−2) and a Bayer-Villager mechanism for the 17,20-lyase reaction42,43. Our data suggest that b5 acts at a microscopic step subsequent to the formation of the peroxo intermediate-heme complex but do not exclude 17,20-lyase reaction mechanisms that involve either compound I or a peroxo-anion.
Mutation T306A shows poor coupling (0.2–6%) with all substrates and disproportionately impaired 17-hydroxylase activity compared to 17,20-lyase activity35, which is already poorly coupled for wild-type P450 17A1. The compromised coupling of the analogous T252A mutation in P450cam has been attributed to reordering of active-site waters and disruption of distal oxygen protonation for the peroxo-heme complex44. In contrast, b5 rescues 38% of the 17,20-lyase activity and 62% of the coupling efficiency compared to wild-type P450 17A1, but only when 17OHPreg is the substrate (Table 4). This apparently substrate-dependent b5-mediated restoration of 17,20-lyase activity suggests differential control of proton delivery to the respective catalytic intermediates, which illustrates the influence of both substrate and b5 on P450-mediated catalysis, as described for P450 4A745. For P450 17A1, the 17-hydroxyls of 17-hydroxysteroid substrates are conspicuously positioned to hydrogen bond directly or indirectly with either the distal or proximal oxygen atoms of the peroxo-heme complex15, which could potentially enhance formation of compound I, direct nucleophillic attack of the peroxo-anion intermediate, promote peroxide formation and uncoupling, or otherwise participate in the reaction cycle. The substrate-dependent effect of b5 on the 17,20-lyase reaction, therefore, is intimately linked to its catalytic mechanism, yet critical details of this process remain elusive.
Nevertheless, the consistent correlation of improved coupling and turnover with b5 for the 17,20-lyase reaction is in agreement with the hypothesis that redox-partner binding on the proximal surface of P450 17A1 may enhance substrate oxidation in the active site on the distal side of the heme. The ~25 crystal structures of P450 2B4 with a variety of substrates bound in the active site support an allosteric linking of the active site and the redox-partner binding site46,47. These structures demonstrate that substrate binding alters the structure of the redox-partner binding site and support the notion that redox partner binding might alter the conformation of the active-site catalytic machinery in the presence of specific substrates. These insights should facilitate the advancement of strategies to selectively target the 17,20-lyase activity of human P450 17A1.
Acknowledgments
We thank Mr. Robert Chomic for assistance with mass spectrometry experiments and Dr. Paul Hollenberg for valuable discussions.
This work was supported by grants R01-GM086596 (to RJA), R01-GM094209 (to LW), and K08-DK1109116 (to AFT) from the National Institutes of Health and by a Veterans Administration Merit Review grant (to LW). Mass spectrometry resources in the Michigan Nutrition Obesity Center (NORC) received support from National Institutes of Health Grant DK089503.
Abbreviations
- 17OHAllo
17-hydroxyallopregnanolone
- 17OHPreg
17-hydroxypregnenolone
- 16OHProg
16α-hydroxyprogesterone
- 17OHProg
17-hydroxyprogesterone
- AD
androstenedione
- Allo
allopregnanolone
- AST
androsterone
- BHT
butylated hydroxytoluene
- Brij
nonaethylene glycol monododecyl ether
- CV
coefficient of variation
- DHEA
dehydroepiandrosterone
- HPLC
high-performance liquid chromatography
- IPTG
isopropyl-1-thio-β-D-galactopyranoside
- MS
mass spectrometry
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced form)
- Ni-NTA
nickel-nitrilotriacetic acid
- NP40
4-nonylphenyl poly(ethylene glycol)
- OD
optical density or absorption at 600 nm
- P450 17A1
cytochrome P450c17
- PBS
phosphate-buffered saline
- POR
cytochrome P450-oxidoreductase
- Preg
pregnenolone
- Prog
progesterone
- TCEP
(2-carboxyethyl)phosphine HCl
- UV
ultraviolet light
References
- 1.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auchus RJ, Lee TC, Miller WL. Cytochrome b 5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 3.Gupta MK, Guryev OL, Auchus RJ. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003;418:151–160. doi: 10.1016/j.abb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Swart P, Swart AC, Waterman MR, Estabrook RW, Mason JI. Progesterone 16α-hydroxylase activity is catalyzed by human cytochrome P450 17α-hydroxylase. J Clin Endocrinol Metab. 1993;77:98–102. doi: 10.1210/jcem.77.1.8325965. [DOI] [PubMed] [Google Scholar]
- 5.Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003;278:48563–48569. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 6.Geller DH, Auchus RJ, Mendonça BB, Miller WL. The genetic and functional basis of isolated 17,20 lyase deficiency. Nature Genet. 1997;17:201–205. doi: 10.1038/ng1097-201. [DOI] [PubMed] [Google Scholar]
- 7.Van Den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. Differential inhibition of 17α-hydroxylase and 17,20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab. 2002;87:5714–5721. doi: 10.1210/jc.2001-011880. [DOI] [PubMed] [Google Scholar]
- 8.Ferraldeschi R, Sharifi N, Auchus RJ, Attard G. Molecular pathways: Inhibiting steroid biosynthesis in prostate cancer. Clin Cancer Res. 2013;19:3353–3359. doi: 10.1158/1078-0432.CCR-12-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE for the COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attard G, Reid AHM, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, Oommen NB, Folkerd E, Dowsett M, Arlt W, de Bono JS. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 12.Auchus ML, Auchus RJ. Human steroid biosynthesis for the oncologist. J Investig Med. 2012;60:495–503. doi: 10.231/JIM.0b013e3182408567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auchus RJ, Yu MK, Nguyen S, Mundle SD. Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer. The oncologist. 2014;19:1231–1240. doi: 10.1634/theoncologist.2014-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–119. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrunak EM, DeVore NM, Porubsky PR, Scott EE. Structures of human steroidogenic cytochrome P450 17A1 with substrates. J Biol Chem. 2014;289:32952–32964. doi: 10.1074/jbc.M114.610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallan PS, Nagy LD, Lei L, Gonzalez E, Kramlinger VM, Azumaya CM, Wawrzak Z, Waterman MR, Guengerich FP, Egli M. Structural and kinetic basis of steroid 17α,20-lyase activity in teleost fish cytochrome P450 17A1 and its absence in cytochrome P450 17A2. J Biol Chem. 2015;290:3248–3268. doi: 10.1074/jbc.M114.627265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onoda M, Hall PF. Cytochrome b 5 stimulates purified testicular microsomal cytochrome P450 (C21 side-chain cleavage) Biochem Biophys Res Commun. 1982;108:454–460. doi: 10.1016/0006-291x(82)90850-6. [DOI] [PubMed] [Google Scholar]
- 18.Naffin-Olivos JL, Auchus RJ. Human cytochrome b 5 requires residues E48 and E49 to stimulate the 17, 20-lyase activity of cytochrome P450c17. Biochemistry. 2006;45:755–762. doi: 10.1021/bi051623y. [DOI] [PubMed] [Google Scholar]
- 19.Peng HM, Liu J, Forsberg SE, Tran HT, Anderson SM, Auchus RJ. Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b 5 . J Biol Chem. 2014;289:33838–33849. doi: 10.1074/jbc.M114.608919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrada DF, Laurence JS, Scott EE. Substrate-Modulated Cytochrome P450 17A1 and Cytochrome b 5 Interactions Revealed by NMR. J Biol Chem. 2013;288:17008–17018. doi: 10.1074/jbc.M113.468926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38:1598–1606. doi: 10.1021/bi9821059. [DOI] [PubMed] [Google Scholar]
- 22.Sandee D, Miller WL. High-yield expression of a catalytically active membrane-bound protein: human P450 oxidoreductase. Endocrinology. 2011;152:2904–2908. doi: 10.1210/en.2011-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bridges A, Gruenke L, Chang YT, Vakser IA, Loew G, Waskell L. Identification of the binding site on cytochrome P450 2B4 for cytochrome b 5 and cytochrome P450 reductase. J Biol Chem. 1998;273:17036–17049. doi: 10.1074/jbc.273.27.17036. [DOI] [PubMed] [Google Scholar]
- 24.Mulrooney SB, Waskell L. High-level expression in Escherichia coli and purification of the membrane-bound form of cytochrome b 5 . Protein Expr Purif. 2000;19:173–178. doi: 10.1006/prep.2000.1228. [DOI] [PubMed] [Google Scholar]
- 25.Guengerich FP, Martin MV. Purification of cytochrome P-450, NADPH-cytochrome P-450 reductase, and epoxide hydratase from a single preparation of rat liver microsomes. Arch Biochem Biophys. 1980;205:365–379. doi: 10.1016/0003-9861(80)90119-8. [DOI] [PubMed] [Google Scholar]
- 26.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 27.Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100:2283–2290. doi: 10.1210/jc.2015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng HM, Auchus RJ. The action of cytochrome b 5 on CYP2E1 and CYP2C19 activities requires anionic residues D58 and D65. Biochemistry. 2013;52:210–220. doi: 10.1021/bi301384n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YH, Tee MK, Miller WL. Human cytochrome P450c17: single step purification and phosphorylation of serine 258 by protein kinase A. Endocrinology. 2010;151:1677–1684. doi: 10.1210/en.2009-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruenke LD, Konopka K, Cadieu M, Waskell L. The stoichiometry of the cytochrome P-450-catalyzed metabolism of methoxyflurane and benzphetamine in the presence and absence of cytochrome b 5 . J Biol Chem. 1995;270:24707–24718. doi: 10.1074/jbc.270.42.24707. [DOI] [PubMed] [Google Scholar]
- 31.Im SC, Waskell L. The interaction of microsomal cytochrome P450 2B4 with its redox partners, cytochrome P450 reductase and cytochrome b 5 . Arch Biochem Biophys. 2011;507:144–153. doi: 10.1016/j.abb.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Gruenke L, Arscott D, Shen A, Kasper C, Harris DL, Glavanovich M, Johnson R, Waskell L. Determination of the rate of reduction of oxyferrous cytochrome P450 2B4 by 5-deazariboflavin adenine dinucleotide T491V cytochrome P450 reductase. Biochemistry. 2003;42:11594–11603. doi: 10.1021/bi034968u. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Im SC, Waskell L. Cytochrome b 5 increases the rate of product formation by cytochrome P450 2B4 and competes with cytochrome P450 reductase for a binding site on cytochrome P450 2B4. J Biol Chem. 2007;282:29766–29776. doi: 10.1074/jbc.M703845200. [DOI] [PubMed] [Google Scholar]
- 34.Tiosano D, Knopf C, Koren I, Levanon N, Hartmann MF, Hochberg Z, Wudy SA. Metabolic evidence for impaired 17α-hydroxylase activity in a kindred bearing the E305G mutation for isolate 17,20-lyase activity. Eur J Endocrinol. 2008;158:385–392. doi: 10.1530/EJE-07-0712. [DOI] [PubMed] [Google Scholar]
- 35.Khatri Y, Gregory MC, Grinkova YV, Denisov IG, Sligar SG. Active site proton delivery and the lyase activity of human CYP17A1. Biochem Biophys Res Commun. 2014;443:179–184. doi: 10.1016/j.bbrc.2013.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geller DH, Auchus RJ, Miller WL. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5 . Mol Endocrinol. 1999;13:167–175. doi: 10.1210/mend.13.1.0219. [DOI] [PubMed] [Google Scholar]
- 37.Simonov AN, Holien JK, Yeung JC, Nguyen AD, Corbin CJ, Zheng J, Kuznetsov VL, Auchus RJ, Conley AJ, Bond AM, Parker MW, Rodgers RJ, Martin LL. Mechanistic scrutiny identifies a kinetic role for cytochrome b5 regulation of human cytochrome P450c17 (CYP17A1, P450 17A1) PLoS One. 2015;10:e0141252. doi: 10.1371/journal.pone.0141252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Idkowiak J, Randell T, Dhir V, Patel P, Shackleton CH, Taylor NF, Krone N, Arlt W. A missense mutation in the human cytochrome b 5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97:E465–E475. doi: 10.1210/jc.2011-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab. 2010;95:994–999. doi: 10.1210/jc.2008-1745. [DOI] [PubMed] [Google Scholar]
- 40.Estrada DF, Skinner AL, Laurence JS, Scott EE. Human cytochrome P450 17A1 conformational selection: modulation by ligand and cytochrome b 5 . J Biol Chem. 2014;289:14310–14320. doi: 10.1074/jbc.M114.560144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17α-hydroxylase-17,20-lyase (CYP17) by cytochrome b 5: endocrinological and mechanistic implications. Biochem J. 1995;308:901–908. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee-Robichaud P, Shyadehi AZ, Wright JN, Akhtar ME, Akhtar M. Mechanistic kinship between hydroxylation and desaturation reactions: Acyl carbon bond cleavage promoted by pig and human CYP17 (P-45017α; 17α-hydroxylase-17,20-lyase) Biochemistry. 1995;34:14104–14113. doi: 10.1021/bi00043a015. [DOI] [PubMed] [Google Scholar]
- 43.Mak PJ, Gregory MC, Denisov IG, Sligar SG, Kincaid JR. Unveiling the crucial intermediates in androgen production. Proc Natl Acad Sci U S A. 2015;112:15856–15861. doi: 10.1073/pnas.1519376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagano S, Poulos TL. Crystallographic study on the dioxygen complex of wild-type and mutant cytochrome P450cam. Implications for the dioxygen activation mechanism. J Biol Chem. 2005;280:31659–31663. doi: 10.1074/jbc.M505261200. [DOI] [PubMed] [Google Scholar]
- 45.Loughran PA, Roman LJ, Miller RT, Masters BS. The kinetic and spectral characterization of the E. coli-expressed mammalian CYP4A7: cytochrome b5 effects vary with substrate. Arch Biochem Biophys. 2001;385:311–321. doi: 10.1006/abbi.2000.2136. [DOI] [PubMed] [Google Scholar]
- 46.Scott EE, He YA, Wester MR, White MA, Chin CC, Halpert JR, Johnson EF, Stout CD. An open conformation of mammalian cytochrome P450 2B4 at 1.6-Å resolution. Proc Natl Acad Sci U S A. 2003;100:13196–13201. doi: 10.1073/pnas.2133986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott EE, White MA, He YA, Johnson EF, Stout CD, Halpert JR. Structure of mammalian cytochrome P450 2B4 complexed with 4-(4-chlorophenyl)imidazole at 1.9-Å resolution: insight into the range of P450 conformations and the coordination of redox partner binding. J Biol Chem. 2004;279:27294–27301. doi: 10.1074/jbc.M403349200. [DOI] [PubMed] [Google Scholar]