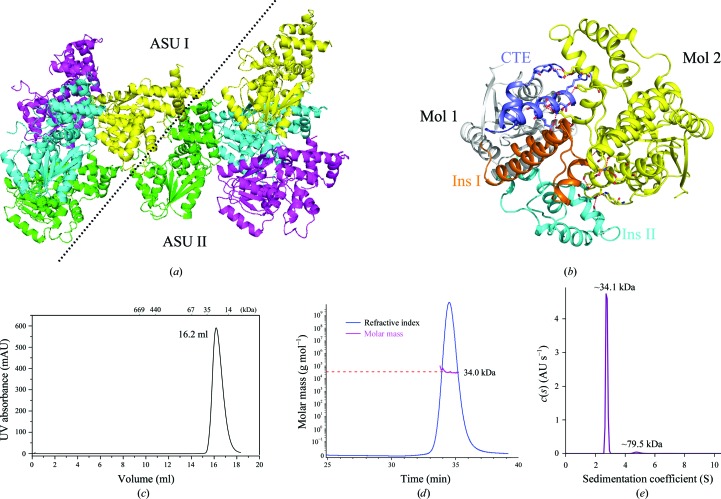
Figure 3.
Fun30 ATPase-C is a monomer in solution. (a) Monomers from neighbouring asymmetric units (ASUs) form tight interactions. The four monomers in an asymmetric unit are in different colours. (b) The interface of the dimer in the crystal. The CTE (blue), Ins I (orange) and Ins II (cyan) of one monomer interact with the other monomer (yellow). Hydrogen bonds are shown as red dashed lines. (c) Size-exclusion chromatogram of Fun30 ATPase-C. The molecular masses of the protein standards are indicated at the top. (d) The mass of Fun30 ATPase-C in solution is about 34.0 kDa, as measured by static light scattering, which corresponds to a monomeric configuration. (e) Analytical ultracentrifugation of Fun30 ATPase-C demonstrates that 95.8% of the total protein, with a molecular mass of about 34.1 kDa, is a monomer. Only 2.6% of the sample shows a molecular mass of 79.5 kDa, which may be an impurity.