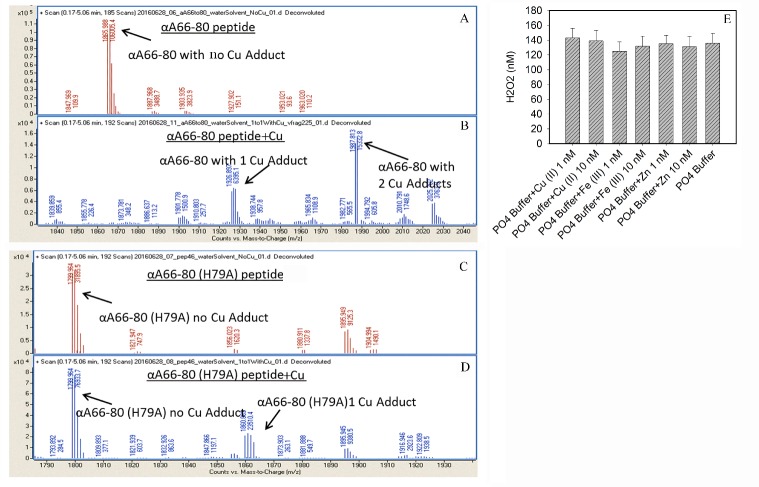
Figure 2.
A-D Nanosprary QTOF spectra of peptides with and without copper in aqueous solution. A) Peptide αA66-80, B) Peptide αA66-80 in presence of excess copper sulphate, C) Peptide αA66-80 (H79A), D) Peptide αA66-80 (H79A) in presence of excess copper sulphate. The αA66-80 peptide can bind upto 2 copper (peak at 1986.81 Da). The two-copper binding peak is suppressed in αA66-80 H79A peptide. The results suggest histidine role in copper binding and subsequent H2O2 generation by αA66-80 peptide. E) Generation of H2O2 by αA66-80 peptide in presence of different metal ions added to 50 mM Phoshpahte buffer, pH 7.2. To test whether addition of metal ions can increase the genration H2O2 by αA66-80 peptide, we incubated the peptide either alone or with Cu (11), Fe (III) or Zn(II), in 1 nM or 10 nM, for 4 hr in PO4 buffer. At the end of incubation, Amplex Red reagent was added and the mixtures were further incubated for 30 min in the dark at room temperature. The fluorescent intensities of the reaction mixtures were measured as mentioned in the method.