Figure 5.
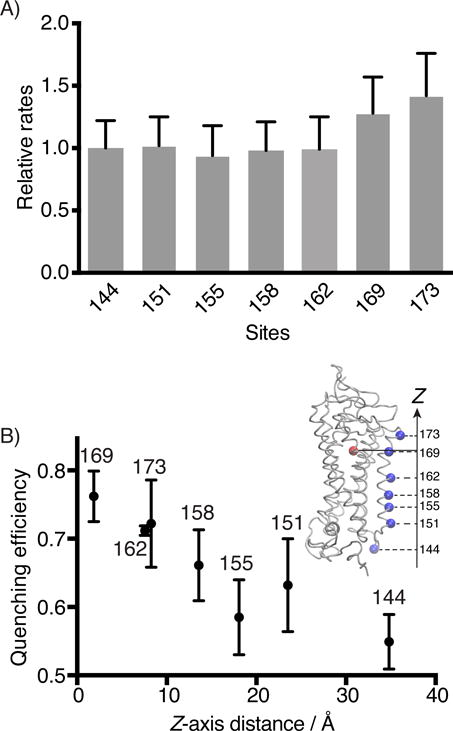
Steady-state fluorescence quenching assay for the binding kinetics of 11-cis-retinal to Alexa488-Rho variants. A) The second-order rate constants (k2) of 11-cis-retinal uptake by different Alexa488-Rho variants. S144-Alexa488 Rho was used as a reference to normalize the reaction rates of other variants. B) The energy transfer efficiencies between 11-cis-retinal (acceptor) and Alexa488 (donor) attached to different sites of Rho plotted versus the Z-axis distance between the alpha carbon of the labeled site (blue dot), and the center of mass of the retinal molecule (red dot), as determined from the crystal structure (inset, PDB 1U19).[55]
