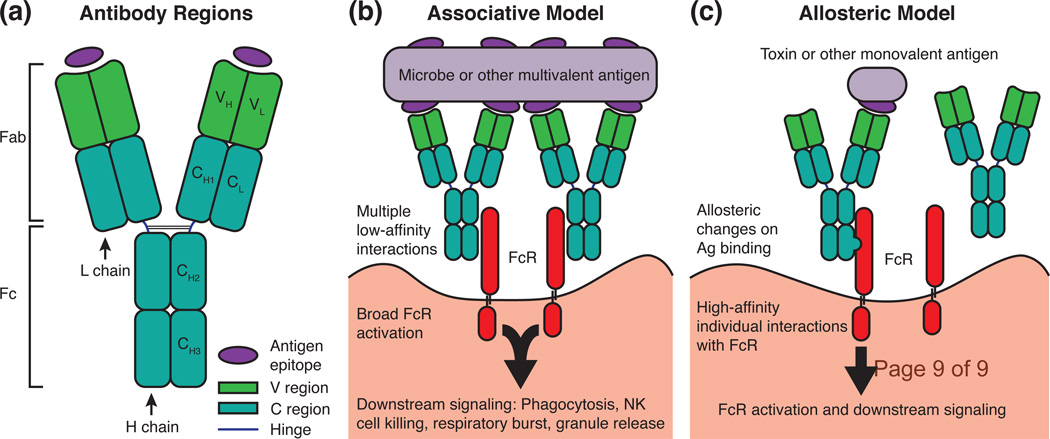
Figure 1. Comparison of the Associative and Allosteric Models of Antibody Signaling.
(a) A simplified schematic of an antibody molecule is shown depicting the H and L chains along with the V and C regions. Immunoglobulins can be broken into two antigen-binding Fab fragments and one Fc fragment. Each Fab contains one combining site that interacts with a single antigen epitope. (b) The associative model of antibody signaling requires multivalent antigens that possess at least two epitopes. The proximity of many antigen (Ag)-bound immunoglobulins leads to many low-affinity interactions between antibody Fc regions and cellular Fc receptors (FcR), ultimately causing FcR activation and downstream signaling. (c) The allosteric, or intramolecular signaling, model can explain FcR activation by antibody bound to monovalent antigens. In this model, antigen binding at the V region causes conformational changes that propagate to the Fc, increasing affinity of the antibody for FcR. Note that the associative and allosteric models of antibody signaling are not mutually exclusive.