Table 1.
Reports of improved ALA delivery using nanotechnology and nanomedicine
| Nanoparticle | Structure | Type of Experiment/Animal Model | Advantages | Clinical Application | Ref |
|---|---|---|---|---|---|
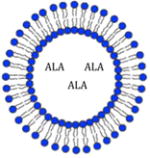
| Liposomes |
|
In vitro and in vivo in rats | Compatible with lipid bilayer structure May employ surface ligands to attach to tissue |
Drug delivery to treat: Skin cancer, Acne, Wrinkles |
[17, 24, 25] |
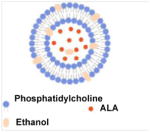
| Ethosomes |
|
Mice | Greater penetration ability than liposomes, smaller particle size | Skin cancer treatment | [21, 26] |
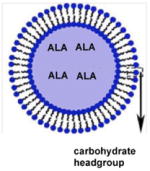
| Niosomes |
|
Excised human skin | 80 and 40% increased permeation of drug and 50 to 100% of the drug retained into the skin when compared to aqueous drug solution | Therapeutic treatment for skin malignancies, hidradenitis suppurativa | [27] |
| Polymeric nanoparticles |
|
HeLa cancer cells Ex vivo skin transport |
10× higher transdermal transport Increased cell killing by PDT |
[28–30] | |
| Mesoporous Silica |

|
Mice | Bypasses the lipophilic barrier to directly enter into cancer cells Exhibited high photocytotoxicity to cancer cells in vitro |
[31] | |
| Conjugated Gold Particles |
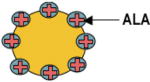
|
Human chronic myeloid leukemia K562 cells, biodistribution in rabbits Human fibroblast and A431 cells |
Used to circumvent multidrug resistance mechanisms Singlet oxygen generation by localized surface plasmon resonance of GNPs |
Early diagnosis and therapy of atherosclerosis | [32–36] |
| Fullerene Nanoparticles |
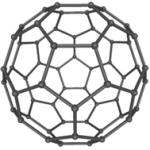
|
Mice | No detectable toxicity Compared to ALA, significant improvement in antitumor efficacy |
[37] | |
| ALA Dendrimers |
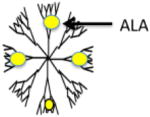
|
LM3 mammary carcinoma cells, PAM 212 murine keratinocyte and A431 human epidermoid carcinoma cell lines | More efficient at low concentrations compared to equimolar ALA for porphyrin production, most lipophilic, minimal dark toxicity, high drug payload | [38–40] | |
| Carbon Nanotubes |
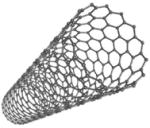
|
MGC-803 tumor cells | Increased PPIX formation Strong optical absorbance in NIR (near-infrared) light |
[41] |
