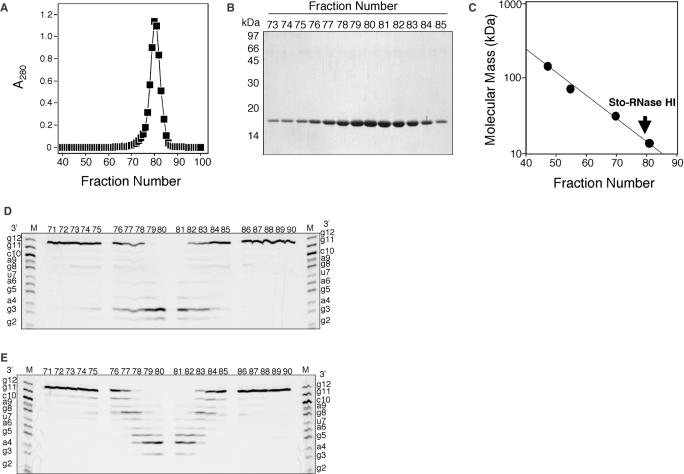
Figure 4.
Gel filtration column chromatography for Sto-RNase HI preparation. (A) Elution profile for gel filtration column chromatography with 20 mM Tris–HCl (pH 8.0) containing 1 mM EDTA and 150 mM NaCl. The flow rate was set at 1.0 ml/min, and 1.0 ml fractions were collected. (B) SDS–PAGE of fractions from the gel filtration column. Numbers along the gel represent the molecular masses of individual standard proteins. (C) Estimation of molecular mass of the Sto-RNase HI. To estimate molecular mass, alcohol dehydrogenase (150 000 Da), BSA (66 000 Da), carbonic anhydrase (29 000 Da) and cytochrome c (12 400 Da) were used as standard proteins. (D) The RNase H activity of each fraction. The fractions were diluted to 1/3000 in reaction mixtures. The reaction conditions were 37°C for 15 min in 10 mM Tris–HCl (pH 8.5) containing 1 mM MnCl2, 10 mM NaCl, 1 mM 2-mercaptoethanol and 50 μg/ml BSA. Product separations were carried out as described in Figure 2. (E) The dsRNase activity of each fraction. The fractions were diluted to 1/100 in reaction mixtures. The reaction conditions and product separations were similar to those described above.