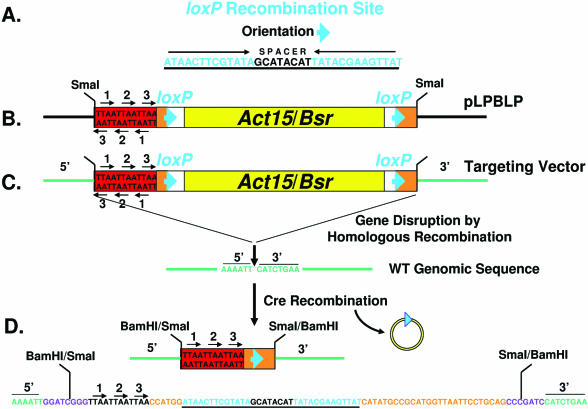
Figure 1.
A strategy for Cre-loxP recycling of the Bsr selectable marker. (A) The loxP recombination site includes an inverted repeat separated by a spacer sequence. (B) The gene targeting vector pLPBLP was constructed with loxP sites in the same orientation flanking both sides of the Bsr expression cassette act15/Bsr. An oligonucleotide cassette was also added that had translational stop codons in all six reading frames. The restriction enzyme sites outside of the floxed-Bsr cassette, such as SmaI (see Materials and Methods), permits the cloning of 5′ and 3′ gene sequences for targeted disruption. (C) The floxed-Bsr cassette of pLPBLP was inserted into the gene DDB0183838 sequence as indicated (see also Figure 2A). A BamHI site was engineered into the targeted gene sequence. This was filled and blunt-end ligated to the SmaI sites flanking the floxed-Bsr cassette to generate the gene-targeting vector. WT cells were transformed for gene disruption by homologous recombination and selected for resistance to Blasticidin S. (D) Transient expression of Cre promotes recombination and deletion of sequences between the two loxP sites in the disrupted gene DDB0183838. A 73 nt sequence remains that includes the translational stop cassette and a single loxP site. The sequence presented was determined directly from a PCR-amplified fragment of the region (see Figure 2B) and matches the predicted recombination event. The disrupted target gene sequences are in green, the loxP site is underlined and the stop codons are indicated by arrows. The site of the BamHI/SmaI fusion is also indicated.