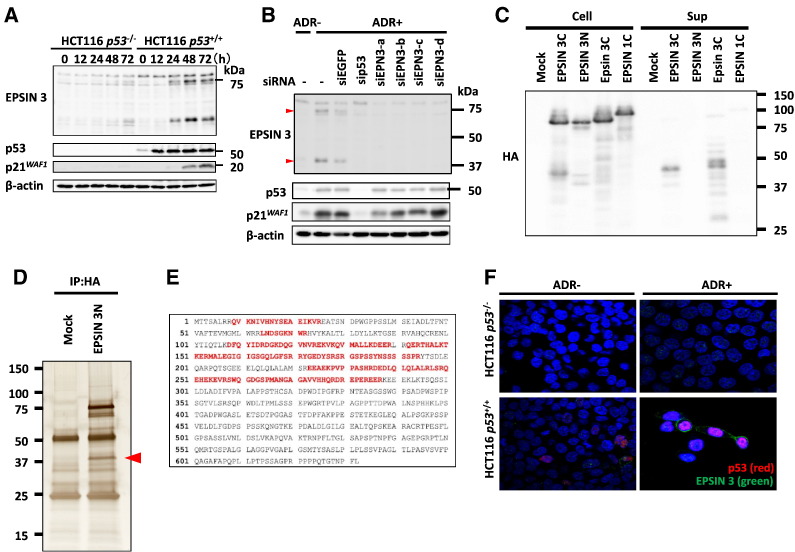
Figure 3.
(A) HCT116 p53−/− and HCT116 p53+/+ cells were treated with 2 μg/ml ADR for 2 h and then incubated in normal medium. At the indicated times after the treatment, whole cell extracts were subjected to immunoblotting with anti-EPSIN 3, anti-p53, anti-p21, or anti β-actin antibodies. (B) We designed four small interfering RNAs (siRNAs): siEPSIN 3-a-d. HCT116 cells were transfected with each siRNA 24 h before treatment with ADR. siEGFP was used as a control. At 48 h after ADR treatment, whole cell extracts were subjected to western blot analysis. Red arrows indicate the protein bands of EPSIN 3. (C) At 24 h after HEK293T cells were transfected with one of the following expression plasmids: EPSIN 3 tagged with HA at its C-terminus (EPSIN 3C), EPSIN 3 tagged with HA at its N-terminus (EPSIN 3 N), mouse Epsin 3 tagged with HA at its C-terminus (Epsin 3C) or EPSIN 1 tagged with HA at its C-terminus (EPSIN 1), the culture medium was replaced with serum-free medium. After another 24 h, the medium was collected, and acetone-precipitated protein samples were subjected to western blot analysis. (D) HEK293T cells were transfected with either EPSIN 3 N or mock vector. At 36 h after transfection, whole cell lysates were subjected to immunoprecipitation with an anti-HA agarose antibody, and the precipitants were analyzed using SDS-PAGE and subsequent silver staining. The red arrow indicates the N-terminal segment of EPSIN 3. (E) The whole amino acid sequence of EPSIN 3 is shown. Red letters indicate peptide sequences detected by mass spectrometry analysis. (F) After incubation with medium containing 0 or 2 μg/ml of ADR for 2 h, HCT116 p53−/− and p53+/+ cells were incubated for 48 h with medium that did not contain ADR. Adherent cells were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature. EPSIN 3 (green) and p53 (red) were visualized using specific primary antibodies and fluorescein-conjugated secondary antibodies. Nuclei were stained with DAPI (blue).