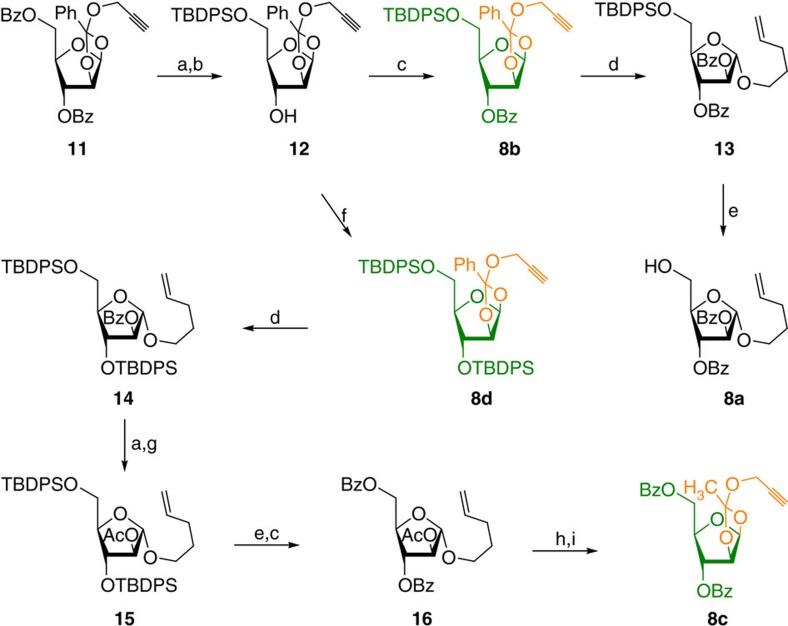
Figure 3. Synthesis of arabinofuranoside-building blocks.
Reagents and conditions: (a) NaOMe, MeOH, 25 °C, 6 h. (b) TBDPS-Cl (1 eq.), imidazole, N,N′-dimethylformamide (DMF), 0→25 °C, 12 h. (c) BzCl, Et3N, CH2Cl2, 0 °C→25 °C, 61%: three steps for 8b from 11. (d) Pent-4-enol, AuCl3, 4 Å MS, CH2Cl2, 25 °C, 2 h. (e) Py·HF, THF:Py (5:1), 0 °C→25 °C, 5 h, 66%: two steps for 8a from 8b. (f) TBDPS-Cl (1 eq.), Imidazole, DMF, 25 °C, 10 h, 74%: two steps for 8d from 11. (g) Ac2O, pyridine, 0 °C→25 °C. (h) Br2, CH2Cl2, 4 Å MS, 0 °C, 15 min. (i) Propargyl alcohol, 2,6-lutidine, CH2Cl2, 4 Å MS, 0 °C →25 °C, 10 h, overall 57% from 8d.