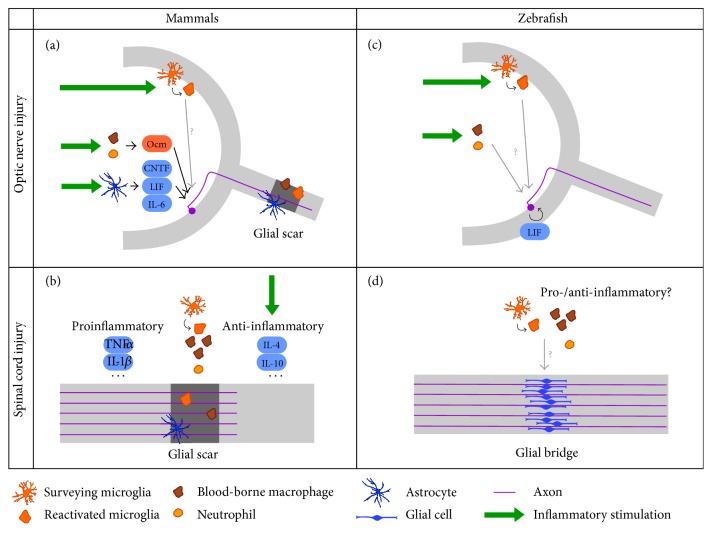
Figure 1.
Summary of the current knowledge of the role of acute inflammation in axonal regeneration, in mammals and zebrafish. (a) After optic nerve injury in mammals, surveying retinal microglia become reactivated, proliferate, and transform into amoeboid microglia. Inflammatory stimulation (green arrows), which can be achieved via administration of TLR2 agonists or lens injury, further induces micro- and macroglial cell activation and an influx of neutrophils and blood-borne macrophages to the vitreous. Infiltrating macrophages and neutrophils secrete oncomodulin (Ocm), an inflammatory mediator that is thought to act on RGCs directly. Moreover, inflammatory stimulation elicits the secretion of IL-6 family cytokines from reactive astrocytes. Signal transduction of these cytokines is primarily mediated via the JAK/STAT3 and mTOR pathways in RGCs. Thus, inflammatory stimulation activates the intrinsic growth state of RGCs, and when combined with SOCS3 and/or PTEN deletion, feedback inhibitors of the JAK/STAT3 and mTOR pathway, respectively, axon regeneration beyond the glial scar can be obtained. (b) After an injury in the mammalian spinal cord microglia are activated, and neutrophils and blood-borne macrophages are recruited to the lesion site. Microglia/macrophages mostly adopt the proinflammatory phenotype and secrete proinflammatory cytokines such as TNF-α and IL-1β, while anti-inflammatory microglia/macrophages, which produce anti-inflammatory cytokines including IL-4 and IL-10, only represent a small percentage. Since the proinflammatory type is associated with adverse effects on regeneration, while anti-inflammatory cells are assumed to be protective and growth-promoting, treatments that stimulate anti-inflammatory activation at the expense of the proinflammatory type (green arrows) improve axonal growth beyond the glial scar and coincide with a better regenerative outcome. (c) Stimulation of acute inflammation after optic nerve injury in zebrafish activates microglia and induces recruitment of neutrophils and blood-borne macrophages, mirroring the situation in mammals. This results in an acceleration of the spontaneous regenerative process. Although it has already been shown that LIF and the JAK/STAT3 and mTOR pathways are implicated in optic nerve regeneration in zebrafish as well, the precise mechanism of how the positive effects of acute inflammation is mediated remains elusive. (d) After a spinal cord injury in zebrafish, microglia are activated and neutrophils and blood-borne macrophages infiltrate the lesion site, although their precise contribution to axonal regeneration is still unknown. Despite pro- and anti-inflammatory macrophages have been described in zebrafish injury models outside the CNS, the polarization of microglia/macrophages after spinal cord injury has not yet been studied. Strikingly, a growth-permissive glial bridge is formed at the lesion site, while glial scar is absent in zebrafish.