FIG 1.
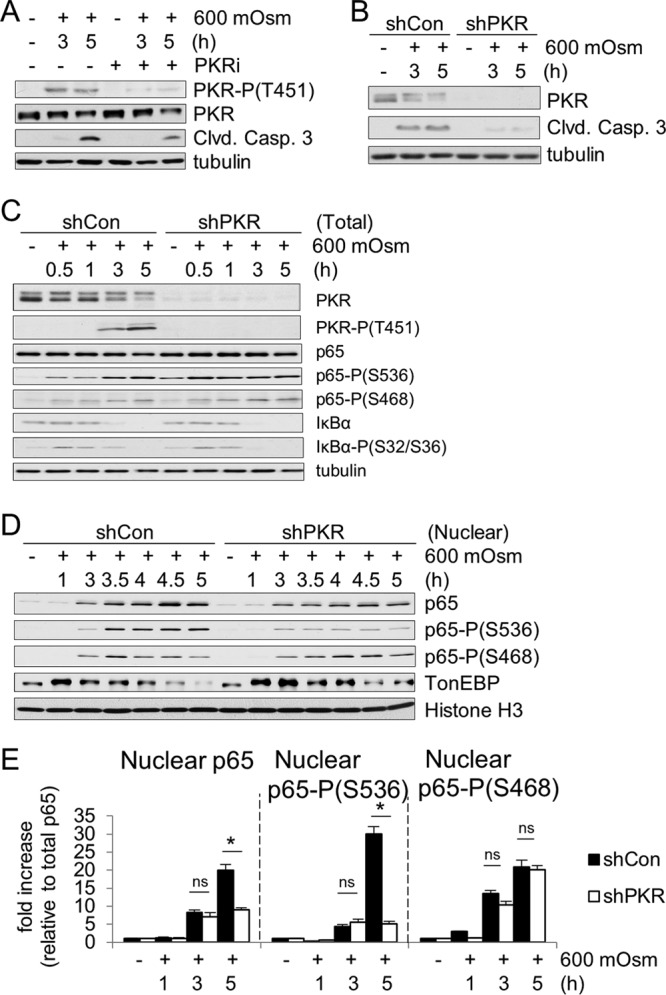
PKR is activated under hyperosmotic stress conditions and affects NF-κB phosphorylation and nuclear localization. (A and B) MEFs were treated with the PKR inhibitor (PKRi; 1.25 μM) for 1 h and then with the addition of hyperosmotic medium (A) or selected for lentivirus infection of control (shCon) or shPKR cells before treatment with hyperosmotic medium (B) for the indicated durations. Lysates were analyzed via immunoblotting for the indicated proteins. (C and D) Total cell lysates and nuclear fractions from control and shPKR MEFs treated with hyperosmotic medium for the indicated durations. Hyperosmotic medium was a final osmolarity of 600 mosM (sucrose). Proteins were detected at the following masses: PKR at 55 kDa; PKR phosphorylated at T451 [PKR-P(T451)] at ∼60 kDa; cleaved (Clvd) caspase-3 at 15 kDa; tubulin at 55 kDa; p65, p65-P(S536), and p65-P(S468) at 65 kDa; IκBα and IκBα-P(S32/S36) at 35 kDa; TonEBP at ∼200 kDa; and histone H3 at ∼20 kDa. (E) Quantification of NF-κB p65, p65-P(S536), and p65-P(S468) translocated to the nucleus (n = 3 biological replicates). Error bars represent standard errors of the means. *, P < 0.05; ns, not significant (Student's t test).
