FIG 5.
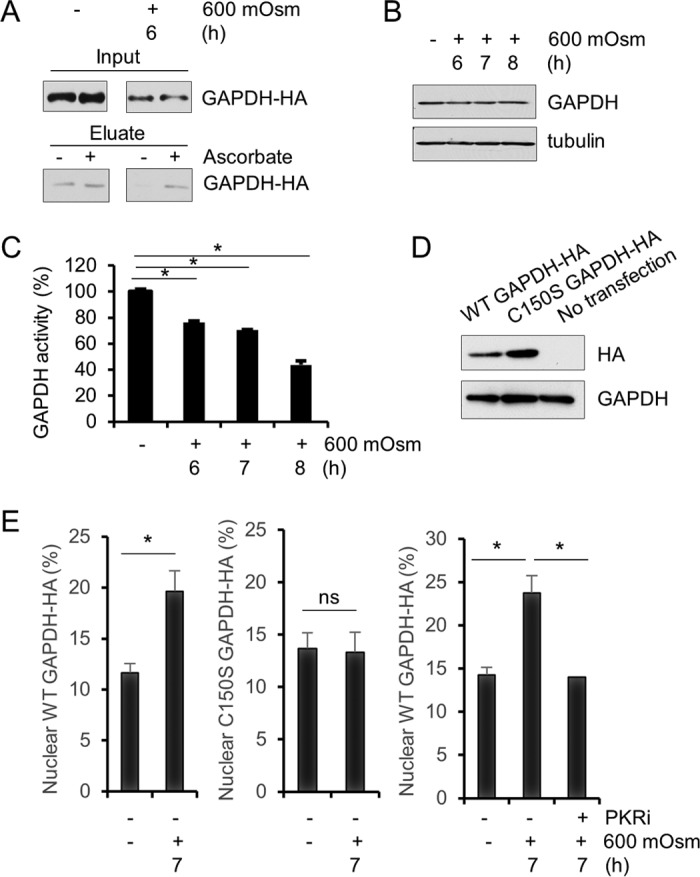
Nitrosylation of GAPDH occurs in hyperosmotic stress. (A) Cell lysates from MEFs treated with hyperosmotic medium for the indicated durations were analyzed for S-nitrosylation in the presence or absence of ascorbate. Eluates were analyzed via immunoblotting. GAPDH protein was detected at ∼35 kDa. (B) Cell lysates from MEFs treated with hyperosmotic medium for the indicated durations were analyzed via immunoblotting for the indicated proteins. (C) GAPDH activity was measured in cell lysates from MEFs treated with hyperosmotic medium for the indicated durations. (D) HA-tagged wild-type and mutant (C150S) GAPDH were expressed in MEFs as verified by Western blotting. HA and GAPDH were detected at ∼35 kDa. (E) Nuclear localization of GAPDH-HA was measured by immunofluorescence and quantified (n = 10) from MEFs transfected with wild-type or mutant GAPDH-HA and treated with hyperosmotic medium and PKRi for the indicated durations. Error bars represent standard errors of the means. *, P < 0.05; ns, not significant (Student's t test).
