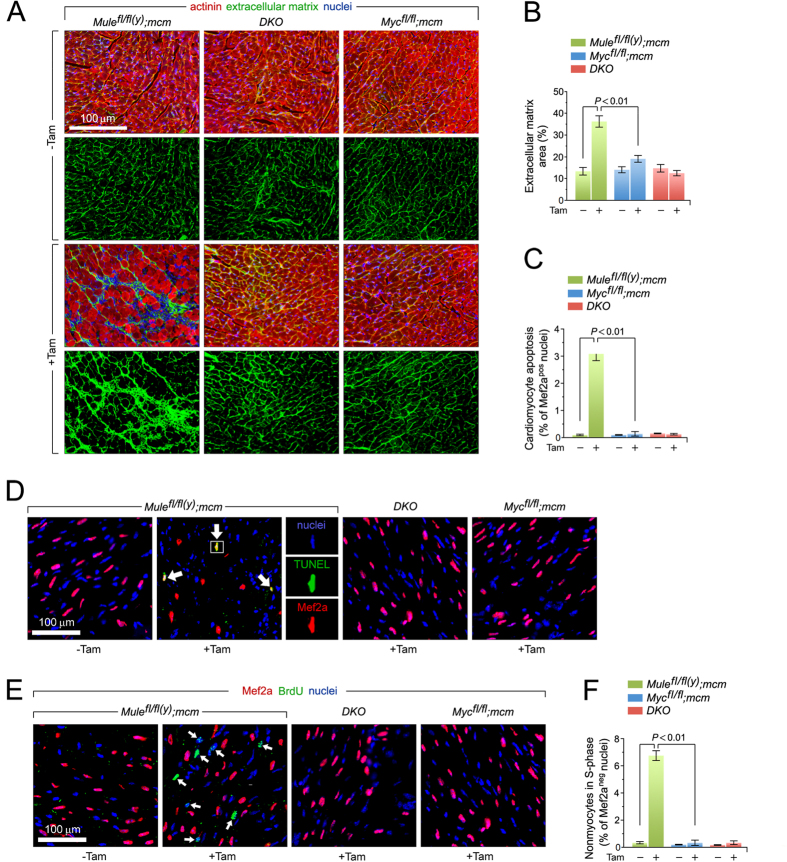
Figure 3. Mule-deficiency induces cardiomyocyte apoptosis and structural remodeling of the ventricular wall.
(A) Analysis of cardiac fibrosis by immunofluorescence microscopy employing wheat germ agglutinin (WGA) staining (green) of collagen deposition in the extracellular matrix, cardiomyocyte-specific anti-actinin (red), and Dapi (blue) to visualize nuclear DNA. (B) Quantification of extracellular matrix area indicative of LV fibrosis shown in (B). n = 4. (C) Acute genetic ablation of Mule triggers cardiomyocyte apoptosis which is abrogated by co- deletion of Mule and Myc in DKO mice. n = 4. (D) Analysis of apoptosis in LV cardiomyocytes (white arrows) by immunofluorescence microscopy and TUNEL assays. Hearts were harvested at 8 d post-Tam. Mice were 12 weeks old at the time of analysis. Red, cardiomyocyte-specific nuclear marker, anti-Mef2a. Green, TUNEL. Blue, DAPI stain of nuclear genomic DNA. TUNEL, terminal deoxynucleotidyl transferase- mediated dUTP nick-end-labeling. (E) BrdU, an indicator for DNA synthesis was injected intraperitoneally at 7 d post-Tam. Animals were sacrificed 18 hours later. Quantitative analysis of cardiomyocytes in S phase was performed by immunofluorescence microscopy of LV cardiac sections employing anti-BrdU (green) and anti-Mef2a (red) antibodies. White arrows denote BrdU-positive and Mef2a-negative non-cardiomyocytes. Blue, nuclei). BrdU, 5-Bromo-2′-deoxyuridine. (F) Genetic ablation of Mule fails to induce cell cycle entry and DNA synthesis in adult cardiomyocytes. n = 4. Data are means ± s.e.m.