Scheme 1.
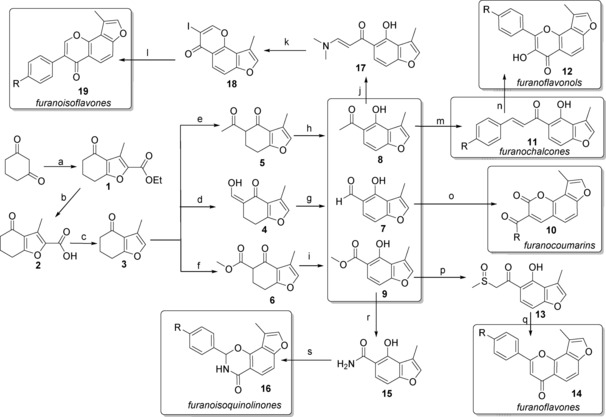
Diversity‐oriented synthesis of 88 compounds based on six natural product frameworks. Reagents and conditions: a) KOH, ethyl 2‐chloroacetoacetate, H2O/MeOH (6:1), RT, 5 days, ≈65 %; b) KOH, H2O/MeOH (1:2.5), RT, 6 h, >90 %; c) Cu, pyridine, DEG, 175 °C, 10 h, ≈85 %; d) NaH, ethyl formate, PhMe, 0 °C–RT, 10 h; e) NaH, ethyl acetate, DME, 0–90 °C, 4.5 h; f) NaH, dimethyl carbonate, DME, 0–90 °C, 4.5 h; g) DDQ, PhMe, 120 °C, 6 h, ≈75 % (d+g); h) DDQ, PhMe, 120 °C, 6 h, ≈70 % (e+h); i) DDQ, PhMe, 120 °C, 6 h, ≈75 % (f+i); j) DMF‐DMA, DMF, 75 °C, 4 h, 99 %; k) I2, CHCl3, RT, 15 h, 90 %; l) arylboronic acid, Na2CO3, Pd(OAc)2, PEG 10 000, MeOH, 50 °C, 4 h; m) benzaldehyde, NaH, THF, RT, 2 h; n) 25 % aq NaOH, 30 % aq H2O2, THF/MeOH (3:5), 0 °C–RT, 48 h; o) reactive methylene compound, piperidine, EtOH, 80 °C, 4 h; p) DMSO, NaH, PhMe, 80 °C, 2 h, 95 %; q) benzaldehyde, piperidine, PhMe, 120 °C, 3 h; r) MeOH (saturated with NH3), 65 °C, 24 h, 100 %; s) benzaldehyde, piperidine, PhMe, 120 °C, 12 h. DEG=diethylene glycol; DDQ=2,3‐dichloro‐5,6‐dicyano‐1,4‐benzoquinone; DMF‐DMA=N,N‐dimethylformamide dimethyl acetal; DME=glycol dimethyl ether; DMF=N,N‐dimethylformamide; PEG=polyethylene glycol; THF=tetrahydrofuran; DMSO=dimethylsulfoxide.
