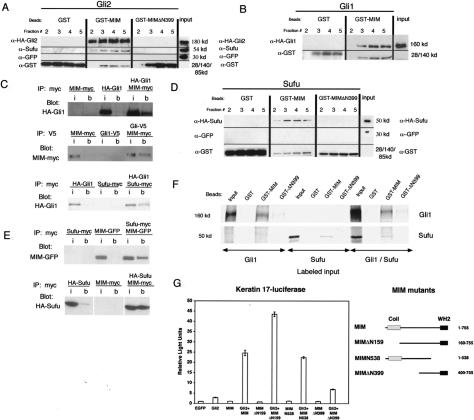
Figure 4.
MIM associates with Gli and Sufu. (A, top row) A MIM column retains HA-Gli2 and endogenous Sufu as seen by retention in eluate fractions. (Middle row) Coexpressed GFP is not retained. (Lower row) GST fusion proteins. Neither GST nor mutant MIMΔN399 retains Gli2 or Sufu. Lines separate portions of the blot probed with different antibodies. The input represents 1% of total 293 cell lysate loaded on column. (B) MIM column also retains HA-Gli1. The input represents 1% of total 293 cell lysate loaded on column. (C) Immunoprecipitated MIM-myc pellets HA-Gli1 in 293 cell lysates. (i) Input; (b) beads. Immunoprecipitated Gli1-V5 pellets MIM-myc in 293 cell lysates. Immunoprecipitated Sufu-myc also pellets HA-Gli1 in 293 cell lysates. The input in each case represents 0.5% of total input. (D, top row) The MIM column retains HASufu as seen by retention in eluate fractions. (Middle row) Coexpressed GFP is not retained. (Lower lane) GST fusion proteins. Neither GST nor MIMΔN399 retains Sufu. The input represents 1% of total eluate loaded. (E) Immunoprecipitated Sufu-myc pellets MIM-GFP and MIM-myc pellets HA-Sufu when coexpressed in 293 cell lysates. (i) Input; (b) beads. The input represents 0.5% of lysate. No binding of GFP to Sufu-myc was detected (data not shown). (F) In vitro translated Gli1 and Sufu bind to GST-MIM. (Top row) The indicated GST beads were incubated with 35S-labeled protein, and the spun pellet was examined by autoradiography. Note no increase in binding of Sufu or Gli to MIM when both are present. The input represents 50% of reaction used. (G) Luciferase activity of BEG4 mutants missing the monomeric actin-binding domain (MIMN538), the F-actin bundling domain (MIMΔN159), or the N-terminal domain required for association with the Gli complex (MIMΔN399). Error bars, ±SEM.