Table 1.
Optimization of the asymmetric allylic alkylation of fluorooxindoles.[a]
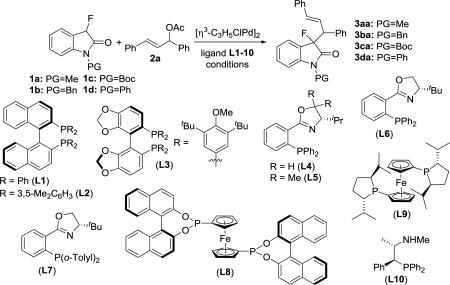
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 1 | ligand | conditions | 3 | yield (%) | dr | ee (%) | |
| major | minor | |||||||
| 1 | 1a | L1 | BSA/NaOAc (3:1), 25 °C | 3aa | 89 | 2.2:1 | 92 | 92 |
| 2 | 1a | L2 | BSA/NaOAc (3:1), 25 °C | 3aa | 88 | 1.5:1 | 86 | 94 |
| 3 | 1a | L3 | BSA/NaOAc (3:1), 25 °C | 3aa | 85 | 1.1:1 | 96 | 93 |
| 4 | 1a | L4 | BSA/NaOAc (3:1), 25 °C | 3aa | 98 | 2.7:1 | 98 | 96 |
| 5 | 1a | L5 | BSA/NaOAc (3:1), 25 °C | 3aa | 99 | 2.7:1 | 98 | 96 |
| 6 | 1a | L6 | BSA/NaOAc (3:1), 25 °C | 3aa | 98 | 3.0:1 | 99 | 98 |
| 7 | 1a | L7 | BSA/NaOAc (3:1), 25 °C | 3aa | 93 | 2.5:1 | 30 | 28 |
| 8 | 1a | L8 | BSA/NaOAc (3:1), 25 °C | 3aa | 98 | 1.1:1 | 68 | 60 |
| 9 | 1a | L9 | BSA/NaOAc (3:1), 25 °C | 3aa | 96 | 1.9:1 | 64 | 72 |
| 10 | 1a | L10 | BSA/NaOAc (3:1), 25 °C | 3aa | 91 | 2.3:1 | 38 | 28 |
| 11 | 1a | L6 | BSA/KOAc (3:1), 25 °C | 3aa | 98 | 3.0:1 | 99 | 99 |
| 12 | 1a | L6 | BSA/Cs2CO3 (3:1), 25 °C | 3aa | 88 | 3.1:1 | 99 | 99 |
| 13 | 1b | L6 | BSA/KOAc (3:1), 25 °C | 3ba | 97 | 3.3:1 | 99 | >99 |
| 14 | 1c | L6 | BSA/KOAc (3:1), 25 °C | 3ca | 96 | 4.1:1 | 99 | 99 |
| 15 | 1d | L6 | BSA/KOAc (3:1), 25 °C | 3da | 93 | 4.7:1 | 99 | >99 |
| 16[b] | 1a | L6 | Et3N, 25 °C | 3aa | 99 | 3.1:1 | >99 | 99 |
| 17[b] | 1a | L6 | iPr2EtN, 25 °C | 3aa | 91 | 2.9:1 | >99 | 99 |
| 18[b] | 1a | L6 | DABCO, 25 °C | 3aa | 64 | 2.4:1 | >99 | 99 |
| 19[b] | 1a | L6 | DBU, 25 °C | 3aa | 95 | 2.5:1 | >99 | 99 |
| 20[b] | 1d | L6 | Et3N, 25 °C | 3da | 99 | 4.7:1 | >99 | 99 |
| 21[b,d] | 1d | L6 | Et3N, 0 °C | 3da | 99 | 7.0:1 | >99 | >99 |
| 22[c,e] | 1d | L6 | Et3N, −10 °C | 3da | 98 | 9.8:1 | >99 | >99 |
| 23[c,f] | 1d | L6 | Et3N, −30 °C | 3da | 96 | >19.0:1 | >99 | >99 |
All reactions were carried out using equimolar amounts of 1 and 2, 5 mol% of the Pd complex and 12 mol% of L1-10 in dichloromethane for 14-18 hours.
2 Equivalents of base.
3 Equivalents of base.
24 h.
36 h.
72 h.
The ee's were determined by chiral chromatography on Chiralpak IA, Amylose I and Cellulose 3. The diastereomeric ratio was obtained from 19F NMR analysis. Boc = tert-butoxycarbonyl, BSA = bis(trimethylsilyl)acetamide, DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DABCO = 1,4-diazabicyclo[2.2.2]octane.
