Abstract
Platelet-derived growth factor (PDGF)-mediated signalling has emerged as one of the most extensively and deeply studied biological mechanism reported to be involved in regulation of growth and survival of different cell types. However, overwhelmingly increasing scientific evidence is also emphasizing on dysregulation of spatio-temporally controlled PDGF-induced signalling as a basis for cancer development. We partition this multi-component review into recently developing understanding of dysregulation PDGF signalling in different cancers, how PDGF receptors are quantitatively controlled by microRNAs. Moreover, we also summarize most recent advancements in therapeutic targeting of PDGFR as evidenced by preclinical studies. Better understanding of the PDGF-induced intracellular signalling in different cancers will be helpful in catalysing the transition from a segmented view of cancer biology to a conceptual continuum.
Keywords: PDGF, signalling, apoptosis, cancer, miRNA
INTRODUCTION
Confluence of information suggested that apoptotic response in different cancers is impaired because of interconnectivity of proteins into signalling networks and complexes that are highly divergent spatio-temporally and promote cell survival. Insights from platelet-derived growth factor (PDGF)-induced signalling research are catalysing new lines of study that should not only explain molecular mechanisms of cancer but also highlight opportunities for targeted therapy. PDGF exists as homodimer and/or heterodimer formed by dimerization of A-polypeptide, B-polypeptide, C-polypeptide and D-polypeptide chains. Hallmark feature of members of PDGF family is that PDGF-AA, PDGF-AB and PDGF-BB are secreted as proteolytically processed molecules from producer cells in secretory vesicles. However, PDGF-CC and –DD are secreted in inactive form. PDGF isoforms transduce the signals intracellularly by binding to PDGFα and β-tyrosine kinase receptors. Structurally, these PDGF receptors are similar, consisting of five immunoglobulin (Ig)-like domains in the extracellular region and tyrosine kinase domains located within the intracellular region. Ig-like domains 2 and 3 offer the binding site for ligand, which is further stabilized by direct receptor–receptor interactions involving Ig-like domain 4. Ten and eleven known autophosphorylated tyrosine residues have previously been identified in platelet-derived growth factor receptor (PDGFR)α and PDGFRβ, respectively, and SH2-domain-containing proteins selectively bind to different phosphorylated residues in the PDGFR, For instance, phospholipase C-γ, SHP-2 tyrosine phosphatase and the GTPase activating protein for Ras have been shown to bind to autophosphoryalted tyroinse residues in PDGFR. PDGFR-mediated phosphorylation of signal transducers and activators of transcription is also an essential characteristic. In addition, PDGFR also provides binding site for proteins, which do not have intrinsic enzymatic activities. Well-studied examples include Grb2, which binds SOS1 to activate Ras and ERK MAP-kinase signalling cascade. p85 regulatory subunit of PI3K also binds to PDGFR that subsequently complexes with the p110 subunit. PDGF-induced signalling is positively and negatively modulated by wide ranging molecules. PDGFR-mediated activation of ERK MAP-kinase is often negatively regulated by MAP-kinase phosphatase 3. However, ubiquitination and degradation of MAP-kinase phosphatase 3 can rescue phosphorylated ERK MAP-kinase to disseminate the signals to downstream effectors.
Although there are some interesting reviews addressing mechanisms that make PDGFR difficult to target,1,2 we emphasize on the most recent developments in our understanding of PDGF-induced signalling and its defects in different cancers. We also provide recent breakthroughs related to quantitative control of PDGFR and PDGF by microRNAs (miRNAs) and how PDGF regulates expression of oncogenic and tumour suppressor miRNAs. It is also well known that PDGFR fuses with different proteins thus adding another layer of intricacy in improving the targeting of PDGFR. We then discuss insights obtained from preclinical studies regarding approaches to slow/retard/inhibit growth of cancer in xenografted mice. Moreover, we provide the landscape of genetic defects in the PDGF family of genes in cancers that is significant.
Defects in PDGF pathway
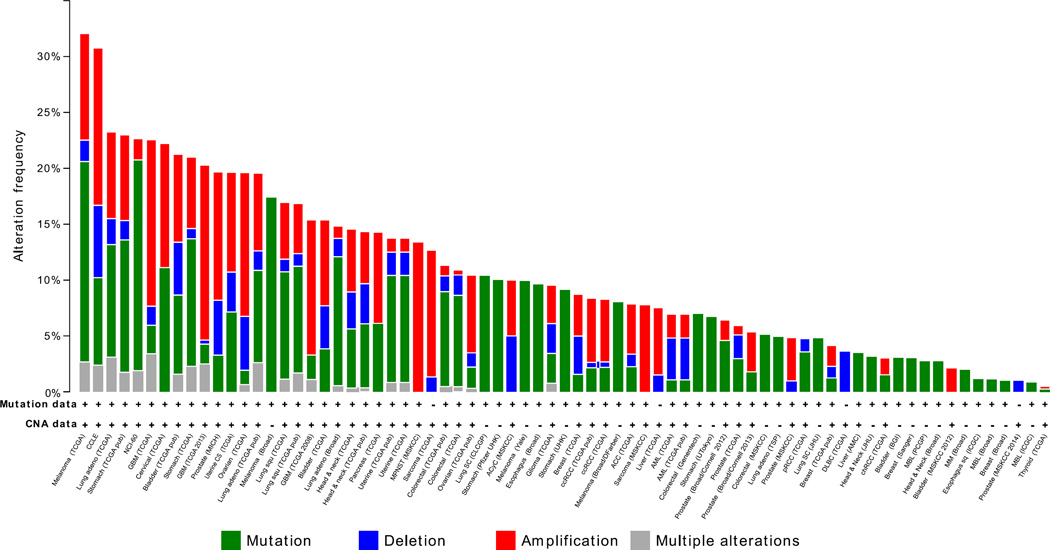
Efforts have been underway in recent years to define genes altered in a variety of cancers. Based on the extensive data from The Cancer Genome Atlas (TCGA) and other organizations (www.cbioportal.org) covering some 80 studies, the incidence of defects, including mutations, deletions and copy number aberrations (CNA), in any one of the PDGF A/B/C/D and PDGFR A/B genes can be observed in as many as 30% of the patients (Figure 1), but this is dependent on the cancer type and the specific study. The highest defects (10% or higher incidence) have been reported in about 30–35 cancer studies investigating a number of cancer types, including melanoma (~10–30%), lung (~10–20%), glioblastoma (~15–20%), bladder (15–20%), prostate (~20%), colorectal (~10–15%), and ovarian (~10–20%). Cancers with consistently lower incidence (<10%) include breast, liver, renal (RCC) and acute myeloid leukaemia. However, these incidences will likely increase in some cases as CNA data become available and added to the database.
Figure 1.
Frequency of alterations in the platelet-derived growth factor (PDGF) family of genes in cancers. The graph indicates frequency of alterations in any of the six genes (PDGA-D and PDFGRA-B) and was compiled following interrogation of about 80 studies in the cBioPortal database
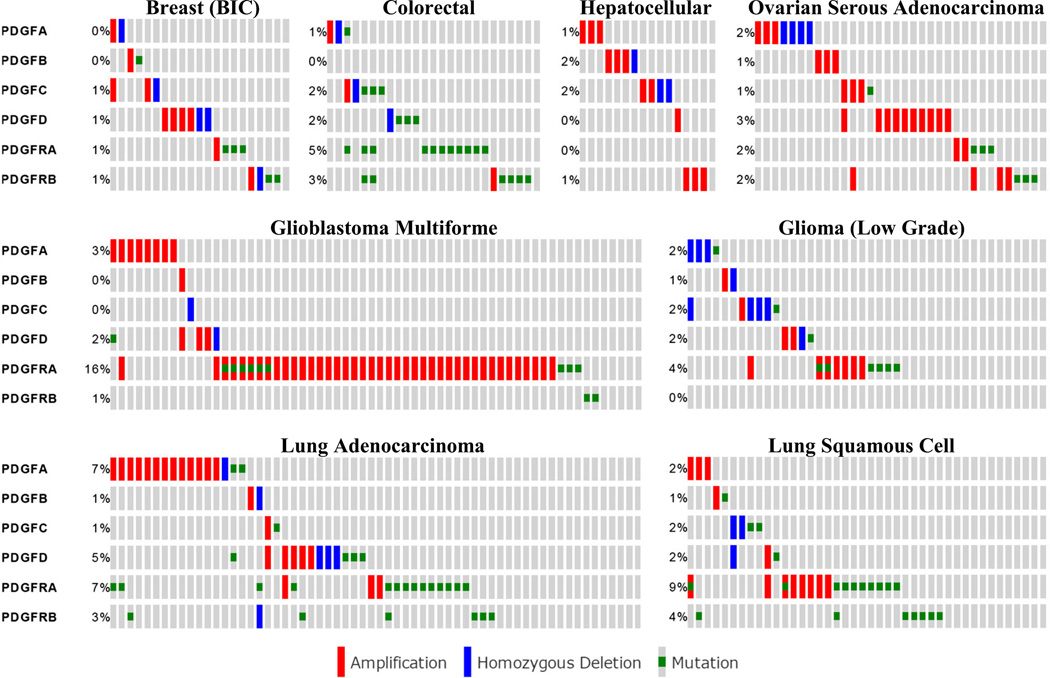
Figure 1 demonstrates defects in any of the six genes of interest but does not indicate the frequency of defects in the individual PDGF ligands and receptors. This has been extracted for selected cancers studied from the TCGA-sponsored studies, and shown in Table 1. In glioblastoma and lung, where the total incidence of PDGF gene defects is about 20–24%, most defects are observed in PDGFR-A (7–16%). Highest incidences in this gene within the PDGF family are also observed in colorectal (5%) and glioma (4%). In breast, liver and ovarian cancers, however, the incidence (0–2%) was lower than for other genes. It is clear that defect in no one particular gene consistently dominates in the deregulation of the PDGF pathway in all cancers. This is consistent with the observation that in 281 cases with defect in any one of the six genes, about a half (54%) were associated with the receptor and the rest (46%) with the ligand. Multiple defects in these genes within the same tumour can also occur, as is apparent from Figure 2. However, the incidence is low for the eight selected cancers, and this suggests that a defect in the single member of PDGF family of genes may be sufficient to disrupt the PDGF pathway.
Table 1.
Distribution of gene alterations in the platelet-derived growth factor (PDGF) family for selected The Cancer Genome Atlas (TCGA)-investigated cancers in the cBioPortal database
| Cases With Altered Gene (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer type | TCGA study id | Total no. of cases |
PDGF-A | PDGF-B | PDGF-C | PDGF-D | PDGFR-A | PDGFR-B | Total |
| Breast invasive carcinoma | Nature 2012 | 482 | 2 (0.4) | 2 (0.4) | 3 (0.6) | 6 (1.2) | 4 (0.8) | 4 (0.8) | 21 (4.2) |
| Colorectal adenocarcinoma | Nature 2012 | 212 | 3 (1.4) | 0 (0) | 5 (2.4) | 4 (1.9) | 11 (5.2) | 7 (3.3) | 30 (14.2) |
| Liver hepatocellular | Provisional | 206 | 3 (1.5) | 4 (2) | 4 (2) | 1 (0.5) | 0 (0) | 3 (1.5) | 15 (7.5) |
| Ovarian serous cystadenocarcinoma | Nature 2011 | 316 | 7 (2.2) | 3 (0.9) | 4 (1.3) | 10 (3.2) | 5 (1.6) | 7 (2.2) | 36 (11.4) |
| Glioblastoma multiforme | Cell 2013 | 281 | 8 (2.8) | 1 (0.4) | 1 (0.4) | 5 (1.8) | 44 (15.7) | 2 (0.7) | 61 (21.8) |
| Glioma (low grade) | Provisional | 262 | 4 (1.5) | 2 (0.8) | 6 (2.3) | 4 (1.5) | 11 (4.2) | 0 (0) | 27 (10.3) |
| Lung adenocarcinoma | Nature, in press | 230 | 16 (7) | 2 (0.9) | 2 (0.9) | 12 (5.2) | 17 (7.4) | 7 (3) | 56 (24) |
| Lung squamous cell | Nature 2012 | 178 | 3 (1.7) | 2 (1.1) | 4 (2.2) | 3 (1.7) | 16 (9) | 7 (3.9) | 35 (19.6) |
| Total | 2167 | 46 (2.1) | 16 (0.7) | 29 (1.4) | 45 (2.1) | 108 (5.0) | 37 (1.7) | 281 (13.0) | |
Figure 2.
Low frequency of multiple gene altered in the platelet-derived growth factor (PDGF) family within the same tumour for selected The Cancer Genome Atlas (TCGA)-investigated cancers. Each cell represents a single case and are aligned vertically to demonstrate occurrence of genetic alterations of any of the six genes within the same tumour sample. Blank cells indicate no alterations were detected. Total number of cases for each cancer can be found in Table 1
Targeting the PDGF pathway in cancers
PDGF pathway and the p53 gene family
A number of unrelated proteins are involved in regulating PDGF signalling, and deregulation of these proteins will upregulate the PDGF pathway. One such regulatory protein is the tumour suppressor p53, which in its wild-type functional sate can transactivate or transrepress a number of target genes. PDGFR is a target, which is transrepressed by p53 to regulate cellular proliferation.3 Mutation of p53 is a common occurrence, observed in about 50% of all cancers, and this may likely be contributing to PDGF-dependent tumorigenesis (Heldin,1). Interestingly, the frequently occurring gain-of-function p53R172H and p53R273H mutants even induce PDGFR-B, which may further promote the aggressive nature of such cancers.4 Similarly, the p53 family member p73 can exist in the ΔNp73 isoform, which has been demonstrated in neuroblastoma to replace wild-type p53 on the PDGFR-B promoter to enhance its expression.5 From a therapeutic perspective, restoring normal p53 function is an obvious option, and small drug molecules in development may provide an opportunity towards this goal. The quinazoline-based small molecules CP-31398 and SCH-529074, for instance, have demonstrated a high capacity to revert gain-of-function p53 mutants to function as wild-type p53. Additional small molecules in this effort to rescue p53 mutants include PRIMA-1, its methylated analogue APR-246, MIRA-1 and STIMA-1, all of which are in preclinical or early clinical development.6–8 Although the potential exists, it remains to be investigated whether such agents will repress PDGF pathway via restoration of p53 function. For therapy against ΔNp73-induced PDGFR-B expression, cisplatin has already been shown to inhibit the growth factor receptor by recruiting p53 and p73 to the PDGFR promoter and in turn displacing the p73 isoform in the IMR-32 neuroblastoma tumour model.5 Whether other cytotoxic drugs, including the platinum-based oxaliplatin, will work in a similar manner remains to be examined.
Brain cancer
MLN0518 (tandutinib) has been noted to cross the blood brain barrier showing notable activity against PDGFRα/β. Intriguingly, doubling time of tumours was remarkably longer in mice xenografted with C6 glioma cells upon treatment with MLN0518 as compared with tumours in vehicle-treated mice.9 CDK4/6 inhibitors have shown potential as anticancer agents. PD-0332991 effectively induced apoptosis in Ink4a-ARF deficient brainstem glioma BSG cell lines as compared with p53 deficient cell lines. PD-0332991-induced cell cycle arrest in mice bearing Ink4a-ARF−/− cells.10 Tumours were bigger in nude mice subcutaneously transplanted with GL261 cells expressing Dkk1 as compared with Wnt1 expressing nude mice. It has been experimentally verified that β-catenin upregulated PDGF-B expression that consequently resulted in marked decline in glioma angiogenesis and normalization of tumour blood vessels.11 MAPK/ERK kinase (MEK) inhibitor U0126 has been reported to potently inhibit cell surface expression of PDGFR-A time dependently. Initially, a decline was noted in phosphorylated levels of ERK; however, phospho-ERK levels were notably enhanced between 3 and 18 h of U0126 treatment in the glioma cell lines.12,13 Downstream of kinase 1 (DOK1) is overexpressed in different glioma cancer cell lines and has been shown to undergo tyrosine phosphorylation in PDGF-BB-treated glioma cells. Detailed mechanistic insights revealed that phosphorylation of p130Cas at tyrosine residues and activation of Rap1 were impaired in DOK1-silenced or DOK1 mutant expressing glioma cells. PDGF-BB treatment induced colocalization of phosphorylated p130Cas and DOK1 at the cell membrane. Mutatnt p130Cas expressing cancer cells did not show Rap1 activation upon treatment with PDGF-BB.14 Dedicator of cytokinesis (Dock180) was noted to be phosphorylated at serine residue 1250 (S1250) in PDGFRα-stimulated glioblastoma cells. S1250 is located within Rac1-binding Dock homology region 2 domain of Dock180, and its phosphorylation resulted in activation of Rac1, p-Akt and ERK 1/2. Mechanistically, it was shown that PDGFRα-induced activation of Dock180 through protein kinase A (PKA)-dependent serine phosphorylation of Dock180. Therefore, effective targeting of PDGFRα-PKA-Dock180-Rac1 signalling axis is necessary.15 PDGFRα overexpression was noted in GBM p-CSC treated with anti-epidermal growth factor receptor (EGFR) therapy. Cell proliferation of p-CSC was notably reduced in PDGFRα-silenced cells while PDGFRα pharmacologically inhibited with Crenolanib resulted in effective targeting of CSC pools.16
Colorectal cancer
Sunitinib mesylate (SU11248, Sutent), an orally bioavailable small molecule, is effective against different tyrosine kinases. Co-injection of colon cancer SW620 cells and colonic fibroblasts in nude mice resulted in significant development of tumour. However, Sunitinib treatment substantially reduced tumour growth in xenografted mice.17–19 PDGFRα promotes transforming growth factor (TGF)-β signalling in hepatic stellate cells via transcriptional and posttranscriptional regulation of TGF-β receptors. Increasingly, it is being recognized that colorectal cancer cell invasion of the liver induces upregulation of PDGFRα of hepatic stellate cells that further promotes TGF-β signalling. PDGFRα silencing inhibited TGF-β-induced intracellular activation and nuclear accumulation of SMAD2. PDGFRα-silenced cells displayed marked increase in TβRII gene transcription. In-depth analysis indicated that TGF-β stimulation promoted recruitment of PDGFRα to TβRI/TβRII complexes to initiate internalization of TβRII. However, TβRII endocytosis and SMAD2 phosphorylation were not noted in PDGFRα-silenced cells. Human colorectal cancer HT29 cells were implanted into the liver of severe combined immunodeficiency mice, and results revealed that HT29 cells colonization in the liver induced activation of HSCs as evidenced by upregulated expression of PDGFRα in HSCs.20,21
Breast cancer
Platelet-derived growth factor has been shown to enhance proliferation of neighbouring luminal MCF-7 breast cancer cells in an oestrogen-independent manner. The findings were obtained from animal model study in which mammary gland stromal cells (BJ3Z) considerably enhanced luminal breast cancer cell proliferation. Mechanistically, it was shown that PDGF ligands secreted by malignant stromal cells signalled through PDGF receptors present on the breast cancer cells to stimulate cellular proliferation.22,23 PDGFRβ expression was upregulated after 2weeks of oestrogen deprivation in oestrogen receptor positive patients. In vitro studies also revealed that long-term oestrogen deprived MCF7 cells expressed higher levels of PDGFRβ.24 Co-culturing breast cancer cell line MDA-MB-231 with tumour-associated macrophages (TAM) induced PDGFRα phosphorylation in TAMs. Additionally, phospho-Akt levels were notably enhanced. Higher apoptosis was noted in TAMs isolated from PDGF-C silenced MDA-MB-231 induced tumour mass.25 BJ3Z are pure malignant mouse mammary stromal cells reportedly involved in enhancing growth of co-cultured breast cancer MCF-7 and BT-474 cells by secretion of PDGF-BB.22,23
Gynaecological cancers
Gene silencing of PDGF-BB in Ca Ski cells enhanced their adherence to endothelial cells; however, adherence was reduced in PDGF-BB silenced HeLa cells.26 Cellular growth and colony forming potential of PDGF-D silenced Ishikawa cells was notably reduced. Moreover, matrix metalloproteinase (MMP2) and MMP9 expression were upregulated in PDGF-transfected endometrial cancer cells.17–19 IMC-3G3, PDGFRα specific monoclonal antibody in combination with docetaxel effectively induced apoptosis in PDGFR overexpressing HeyA8-MDR ovarian cancer cells. Tumour growth was also inhibited in mice xenografted with HeyA8-MDR cells upon treatment with IMC-3G3 and docetaxel.27 Nilotinib alone and in combination with paclitaxel and carboplatin induced apoptosis in PDGFRα-expressing ovarian cancer cells.28
Lung cancer
Non-small cell lung cancer (NSCLC) patients who had overexpression of both PDGF-BB and VEGF-C presented with higher tumour growth and lymphatic invasion.20,21 Crenolanib has been shown to be effective against PDGFR and induced apoptosis in NSCLC A549 cells. Moreover, Crenolanib significantly inhibited tumour growth in nude mice injected with A549 cells injected axillary regions.17–19
CP-673451 also efficiently inhibited PDGFR-induced intracellular signalling and induced apoptosis in A549 cells. Tumour growth was markedly reduced in xenografted mice after treatment with CP-673451.29
Osteosarcoma
Co-culture of platelets with osteosarcoma cells (HOS or MG63) induced PDGFR phosphorylation. Sunitinib or LY294002 notably reduced PDGF-induced PDGFR phosphorylation in osteosarcoma cells.30 TRAIL efficiently induced apoptosis in PDGFRβ-silenced Ewing sarcoma cells. Imatinib-treated and TRAIL-treated xenografted mice did not develop spontaneous lung metastases.31
Hepatocellular carcinoma
Co-culturing hepatic stellate cells with HepG2 cells remarkably enhanced invasive ability of HepG2 cells. However, sorafenib dramatically reduced HepG2 cell invasion.32 Targeted inhibition of PDGF-D in gemcitabine-resistant HCC cells partially induced reversal of EMT phenotype.33
Apoptosis inducing activity of PDGF
Cholangiocarcinoma cells abundantly expressed PDGF-B and PDGF-D, and surprisingly, both isomers facilitated cell death in myofibroblasts upon treatment with BH3 mimetics. PDGF-induced apoptosis in cancer-associated fibroblasts via Puma-mediated Bak activation. PDGF did not induce apoptosis in Puma-silenced cells.34,35
miRNA regulation of PDGFR
Certain hints have emerged suggesting that PDGF and its receptors are quantitatively controlled by miRNAs. Moreover, PDGF has also been shown to modulate expression of miRNA.
miR-34a has recently been shown to effectively inhibit tumourigenic potential of AGS cancer cell line via negative regulation of PDGFR and MET. Mechanistically, it was shown that pAkt played an essential role in transducing the signals intracellularly. miR-34a-mediated repression of PDGFR and MET considerably reduced pAkt levels in AGS cells.36 There is an exciting piece of evidence suggesting that miR-34a negatively regulates PDGFRβ in cultured rat mesangial cells.12,13 Likewise, NSCLC cells have downregulated miR-34a/c and upregulated PDGFRα/β that impaired TRAIL-mediated apoptosis. It was further suggested that gene silencing of PDGFRα/β or overexpression of miR-34a/c in NSCLC cells restored TRAIL-induced apoptosis.37
It has previously been indicated that PDGF-induced intracellular signalling repressed miR-34a expression in proneural glioma cells.38 miR-21 expression was downregulated in PDGF-BB-silenced cancer cells. There was a fivefold increase in the apoptotic rate after silencing of miR-21 both in the human glioblastoma cell line LN18 and in an p16Ink4a/p19Arf double knockout mouse glioma cell culture.39
Regulators of PDGFR
Expression of PDGFRβ is reported to be controlled by Prox1 transcription factor. Prox1 silenced human dermal LECs had notably reduced PDGFRβ expression that inhibited migratory potential of human dermal LECs towards PDGF-BB.40 Lymphoma development and tumour dissemination in a mouse model of NPM-ALK-induced lymphomagenesis is reported to be triggered by JUN-mediated and JUNB-mediated transcriptional upregulation of PDGFRβ.41 However, this molecular mechanism was not noted in NSCLC patients.42 There is a direct piece of evidence emphasizing on the fact that EGFR inhibitor erlotinib only modestly inhibited growth of EGFRvIII expressing U87 glioma cells in xenografted mice. Immunoblots of tumour lysates revealed that PDGFRβ was upregulated and displayed kinase activity in EGFRvIII-silenced cells. Moreover, erlotinib-induced transcriptional upregulation of PDGFRβ was notably impaired in cells ectopically expressing constitutively active AKT1. Similarly, erlotinib-induced PDGFRβ expression was also impaired in constitutively active mTOR expressing cells. However, PDGFRβ expression was markedly enhanced in Raptor-silenced and Rictor-silenced cells.43,44
PDGFR fusion proteins
FIP1-like 1 (FIP1L1)-PDGFRα is a fused transcript aberrantly generated by an 800-kb cryptic interstitial deletion in chromosome 4q12 that encodes constitutively active and a ligand-independent tyrosine kinase. T674I FIP1L1-PDGFRα mutation has been shown to block the access of therapeutic drugs to a hydrophobic pocket inside the ATP binding site. Treating T674I FIP1L1-PDGFRα-expressing BaF3 cells with Ponatinib considerably reduced receptor phosphorylation and its downstream effectors including Stat5, Stat3, ERK1/2 and Akt. Ponatinib significantly reduced tumour growth in BALB/c mice injected with BaF3-T674I PDGFRα cells subcutaneously.45 T674I FIP1L1-PDGFRα was also targeted effectively by DCC-2036, a third-generation TKI as evidenced by reduced receptor phosphorylation and decrease in phosphorylated levels of its downstream substrates. DCC-2036 inhibited tumour growth in nude mice xenografted with BaF3 cells expressing FIP1L1-PDGFRα. Mechanistically, it was shown that DCC-2036-induced apoptosis of FIP1L1-PDGFRα positive cells primarily through increase in Bim-EL expression. Untreated cells had higher levels of phosphorylated Bim-EL and phospho-ERK 1/2. PDGFRα inhibition dramatically reduced phospho-ERK 1/2 levels, and consequently, phospho-ERK 1/2 mediated inhibitory effects on Bim-EL via its phosphorylation and consequent polyubiquitination.46 Tyrosine-kinase domain of PDGFR-B is retained in CCDC88C-PDGFR-B and DTD1-PDGFR-B fusion genes, and coiled-coil domains of fusion partners constitutively activated PDGFR-B fusion protein.47 C-terminal PDGFRα can undergo homodimerization by itself thus showing constitutive kinase activity. C-terminal PDGFRα portion and full-length PDGFRα in FIP1L1-PDGFRα revealed differential activity in IL-3-dependent haematopoietic BAF-B03 cells. Growth of full-length FIP1L1-PDGFR-A expressing BAF-B03 cells was IL-3 independent.48 Phe to Ser exchange is noted in FIP1L1-PDGFR-A at position 604 (F604S) that results in creation of a binding site for the phosphatase domain of SHP-2 leading to considerably reduced autophosphorylated levels of FIP1L1-PDGFR-A/F604S. Significant reduction in activation of SRC and CBL by FIP1L1-PDGFR-A/F604S is also an associated mechanism that stabilizes FIP1L1-PDGFR-A as evidenced by stable protein levels of FIP1L1-PDGFR-A in SRC-inhibited and/or SRC-silenced cells.49
Pre-clinical studies
Lenvatinib mesilate (lenvatinib) a multiple RTK inhibitor exerted its inhbitory effects on PDGFRα tyrosine kinase.50
It has been convincingly revealed that intraperitoneally administered imatinib and transducing PDGFRβ/Fc chimaera expressing adenoviral system in mice significantly repressed inflammatory lymphangiogenesis. Moreover, tumour lymphangiogenesis was reduced in a BxPC3 pancreatic cancer xenograft model upon expression of PDGFRβ/Fc.40 Imatinib mesylate did not inhibit tumour growth in mice subcutaneously transplanted with PDGFR-β overexpressing malignant peripheral nerve sheath NMS-2PC cells.51
Cisplatin resistant testicular germ cell tumour cells displayed markedly increased PDGFRβ and PDGF-B ligand expression. Genetic or chemical inhibition of PDGFRβ restored sensitivity to cisplatin in resistant phenotype.52
Severe combined immunodeficiency mice xenograftted with EGFRvIII expressing U87 glioma cells demonstrated a modest decrease in tumour growth upon treatment with erlotinib; however, significant growth rate was maintained by tumours. Data obtained from phospho-receptor tyrosine kinase array on erlotinib-treated tumour lysates revealed that phospho-PDGFRβ levels were remarkably higher in EGFRvIII-inhibited group. In vitro analysis also confirmed that erlotinib-induced PDGFRβ expression was impaired in cells ctopically expressing AKT1. PDGFRβ expression was upregulated in mTORC1-silenced cells. However, erlotinib-induced PDGFRβ expression was also abrogated in cell expressing constitutively active mTOR. In vivo study emphasized on the fact that PDGFRβ silencing effectively inhibited tumour growth in mice xenografted with U87 cells expressing kinase-dead EGFRvIII.43,44
Platelet-derived growth factor receptors were detectable in infiltrating stroma but not in tumour cells or vessels and Sorafenib treatment considerably eliminated all blood vessels. Ligand-induced PDGFRα stimulation intracellularly activated downstream effectors including Akt, GSK-3α and GSK-3β. PDGFR-selective inhibitor CP-673,451 treatment efficiently decreased PDGFRα-induced activation of Akt, GSK-3α and GSK-3β.53
It is noteworthy that SDF-1α stimulated expression of PDGF-B in vivo in TC/siVEGF7-1 tumours. Bone marrow cells (BMC) express PDGFRβ; however, desmin and NG2 are expressed by mature pericyte only. PDGFRβ +BMCs cultured in PDGF-B containing medium differentiated into pericytes. Mice Intratumorally injected with SDF-1α gene expressing adenoviral vector displayed a marked increase in BM-derived pericytes surrounding the tumour vessels. CXCR4 antagonist AMD 3100 also considerably inhibited BMCs differentiation and induced apoptosis in xenografted mice.54
Platelet-derived growth factor-R inhibitor nilotinib has recently been tested for efficacy in an orthotopic mouse model of human colon cancer and a model of liver metastasis. Results revealed that stromal reaction of xenografts growing in the cecal wall and liver was significantly decreased when individually treated with nilotinib. There was a considerable reduction in stromal reaction and tumour cell proliferation. Moreover, apoptotic cell death of tumour cells increased significantly that resulted further in tumour growth inhibition at both the orthotopic and the metastatic site in animal model combinatorially treated with nilotinib and mTOR inhibitor everolimus.55
There is evidence that co-culturing of WI-38 fibroblasts and hepatocellular carcinoma cells induced activation of PDGFRα in WI-38. Active WI-38 cells consequently increased spheroid formation capacity of HCC cells. TSU-68 is a multikinase inhibitor effectively inhibited PDGFRα phosphorylation in WI-38 cells and tumour growth in mice subcutaneously co-injected with HuH7/WI-38.56
Platelet-derived growth factor-B considerably inhibited cell invasion and tumour metastasis in xenografted mice.57
Clinical trials
Rapamycin-mediated mTORC1 inhibition considerably enhanced PDGFRα-induced Akt phosphorylation. It is also surprising to note that PDGFRα overexpressing synovial sarcoma cells displayed significant response to imatinib-mediated inhibitory effects on rapamycin-induced phospho-Akt. Akt was inhibited in PDGFRα positive, recurrent, metastatic synovial sarcoma patients treated with imatinib and mTORC1 inhibitor everolimus.58 It is now known that fusion genes involving PDGFR-B result in constitutive tyrosine kinase activity in patients with PDGFR-B rearrangements and are sensitive to imatinib.59
Dasatinib (BMS-354825) was tested for efficacy in a phase II, single-arm study in patients with metastatic pancreatic adenocarcinoma however failed to show clinical activity as first-line therapy.60
In a recently reported multicenter phase II clinical trial of pazopanib in progressive and metastatic medullary thyroid carcinoma patients, pazopanib displayed promising clinical activity.61
In advanced gastrointestinal stromal tumour patients, although pazopanib was well tolerated, but it displayed marginal activity.62 Data obtained from phase I clinical trial of dovitinib indicated clinical benefit in patients who had failed prior mTOR inhibitor and VEGF-targeted therapies.63
Olaratumab, an anti-PDGFRα monoclonal antibody was noted to be effective when administered as evidenced by best response of stable disease in 12 patients. Recommended phase II dosages were 20 mg/kg biweekly and 16 mg/kg weekly.64 Immunohistochemical analysis of post-lapatinib-treated patient showed significant reduction of phospho-EGFR and considerably enhanced PDGFRβ expression.43,44
CONCLUSION
There is progressive enrichment in PDGF-induced signalling landscape, but there are some knowledge gaps which have to be bridged related to therapeutic targeting of PDGFR in different cancers. Recently, it has been shown that a patient having defective PDGFR-A, KIT, FBXW7 KRAS, APC and ERB4 genes responded partially to dual m-TORC 1/2 inhibitor, AZD2014 in a first-in-human pharmacokinetic and pharmacodynamic study.64 There is a need to identify PDGFR gene mutations in subgroup of patients, which are less aggressive and have a good prognosis as evidenced by a study reporting that patients with mutations in exon 18 of the PDGFR gene had a 5-year OS (84.6%).66 How PDGF mediates expression of oncogenic and tumour suppressor miRNAs context dependently and how different miRNAs quantitatively control PDGFR are some of the unexplored aspects. Moreover, how PDGF signalling can be targeted to induce apoptosis in cancer cells using natural agents also needs extensive research work.
Acknowledgments
This study was supported in part by the US Public Health Service NCI grant CA160687.
LIST OF ABBREVIATIONS
- DOK1
downstream of kinase 1
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal regulated kinase
- MMP
matrix metalloproteinase
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PKA
protein kinase A
- SMAD
SMA and mothers against decapentaplegic (MAD), Drosophila, homolog
- TGF
transforming growth factor
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict if interest.
REFERENCES
- 1.Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal. 2013b;11:97–114. doi: 10.1186/1478-811X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y. Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol Med. 2013;19(8):460–473. doi: 10.1016/j.molmed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Yang W, Wetterskog D, Matsumoto Y, Funa K. Kinetics of repression by modified p53 on the PDGF beta-receptor promoter. Int J Cancer. 2008;123(9):2020–2030. doi: 10.1002/ijc.23735. [DOI] [PubMed] [Google Scholar]
- 4.Weissmueller S, Manchado E, Saborowski M, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell. 2014;157(2):382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wetterskog D, Moshiri A, Ozaki T, et al. Dysregulation of platelet-derived growth factor β-receptor expression by ΔNp73 in neuroblastoma. Mol Cancer Res. 2009;7(12):2031–2039. doi: 10.1158/1541-7786.MCR-08-0501. [DOI] [PubMed] [Google Scholar]
- 6.Lane DP, Chit FC, Sonia L. p53-based cancer therapy. Cold Spring Harb Perspect Biol. 2010;2:a001222. doi: 10.1101/cshperspect.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann BD, Pietenpol JA. Targeting mutant p53 in human tumors. J Clin Oncol. 2012;30(29):3648–3650. doi: 10.1200/JCO.2012.44.0412. [DOI] [PubMed] [Google Scholar]
- 8.Siddik ZH. Apoptosis in cancer: mechanisms, deregulation, and therapeutic targeting. In: Neidle S, editor. Cancer Drug Design and Discovery. 2nd. Elsevier; 2014. pp. 357–390. [Google Scholar]
- 9.Boult JK, Terkelsen J, Walker-Samuel S, Bradley DP, Robinson SP. A multi-parametric imaging investigation of the response of C6 glioma xenografts to MLN0518 (tandutinib) treatment. PLoS One. 2013;8(4e63024) doi: 10.1371/journal.pone.0063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton KL, Misuraca K, Cordero F, et al. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS One. 2013;8(10):e77639. doi: 10.1371/journal.pone.0077639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis M, Czupalla CJ, Ziegler N, et al. Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J Exp Med. 2012;209(9):1611–1627. doi: 10.1084/jem.20111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Li Y, Mei Y, et al. miR-34a regulates mesangial cell proliferation via the PDGFR-β/Ras-MAPK signaling pathway. Cell Mol Life Sci. 2014a;71(20):4027–4042. doi: 10.1007/s00018-014-1599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Zuo D, Luan C, et al. Glioma cell proliferation controlled by ERK activity-dependent surface expression of PDGFRA. PLoS One. 2014b;9(1e87281) doi: 10.1371/journal.pone.0087281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett A, Evans IM, Frolov A, et al. A crucial role for DOK1 in PDGF-BB-stimulated glioma cell invasion through p130Cas and Rap1 signalling. J Cell Sci. 2014;127(Pt 12):2647–2658. doi: 10.1242/jcs.135988. [DOI] [PubMed] [Google Scholar]
- 15.Feng H, Li Y, Yin Y, et al. Protein kinase A-dependent phosphorylation of Dock180 at serine residue 1250 is important for glioma growth and invasion stimulated by platelet derived growth factor receptor α. Neuro Oncol. 2014:pii: nou323. doi: 10.1093/neuonc/nou323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cenciarelli C, Marei HE, Zonfrillo M, et al. PDGF receptor alpha inhibition induces apoptosis in glioblastoma cancer stem cells refractory to anti-Notch and anti-EGFR treatment. Mol Cancer. 2014;13:247. doi: 10.1186/1476-4598-13-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Song L, Ge H, et al. Crenolanib, a PDGFR inhibitor, suppresses lung cancer cell proliferation and inhibits tumor growth in vivo. Onco Targets Ther. 2014a;7:1761–1768. doi: 10.2147/OTT.S68773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Qiu H, Hu W, Li S, Yu J. Over-expression of platelet-derived growth factor-D promotes tumor growth and invasion in endometrial cancer. Int J Mol Sci. 2014b;15(3):4780–4794. doi: 10.3390/ijms15034780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZH, Li Q, Ruan SQ, et al. Sunitinib mesylate inhibits proliferation of human colonic stromal fibroblasts in vitro and in vivo. J Zhejiang Univ Sci B. 2014c;15(8):701–712. doi: 10.1631/jzus.B1300306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Li J, Xiang X, et al. PDGF receptor-α promotes TGF-β signaling in hepatic stellate cells via transcriptional and posttranscriptional regulation of TGF-β receptors. Am J Physiol Gastrointest Liver Physiol. 2014a;307(7):G749–G759. doi: 10.1152/ajpgi.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Liu C, Qiu L, Li J, Zhang P, Sun Y. Overexpression of both platelet-derived growth factor-BB and vascular endothelial growth factor-C and its association with lymphangiogenesis in primary human non-small cell lung cancer. Diagn Pathol. 2014b;9:128. doi: 10.1186/1746-1596-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto MP, Dye WW, Jacobsen BM, Horwitz KB. Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling. BMC Cancer. 2014a;14(1):735. doi: 10.1186/1471-2407-14-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto MP, Dye WW, Jacobsen BM, Horwitz KB. Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling. BMC Cancer. 2014b;14:735. doi: 10.1186/1471-2407-14-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigel MT, Ghazoui Z, Dunbier A, Pancholi S, Dowsett M, Martin LA. Preclinical and clinical studies of estrogen deprivation support the PDGF/Abl pathway as a novel therapeutic target for overcoming endocrine resistance in breast cancer. Breast Cancer Res. 2012;14(3):R78. doi: 10.1186/bcr3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son D, Na YR, Hwang ES, Seok SH. Platelet-derived growth factor-C (PDGF-C) induces anti-apoptotic effects on macrophages through Akt and Bad phosphorylation. J Biol Chem. 2014;289(9):6225–6235. doi: 10.1074/jbc.M113.508994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tudoran OM, Soritau O, Balacescu L, et al. PDGF beta targeting in cervical cancer cells suggest a fine-tuning of compensatory signalling pathways to sustain tumourigenic stimulation. J Cell Mol Med. 2014 doi: 10.1111/jcmm.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuo K, Nishimura M, Komurov K, Shahzad MM, Ali-Fehmi R, Roh JW, et al. Platelet-derived growth factor receptor alpha (PDGFRα) targeting and relevant biomarkers in ovarian carcinoma. Gynecol Oncol. 2014;132(1):166–175. doi: 10.1016/j.ygyno.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weigel MT, Rath K, Alkatout I, et al. Nilotinib in combination with carboplatin and paclitaxel is a candidate for ovarian cancer treatment. Oncology. 2014;87(4):232–245. doi: 10.1159/000363656. [DOI] [PubMed] [Google Scholar]
- 29.Xi Y, Chen M, Liu X, Lu Z, Ding Y, Li D. CP-673451, a platelet-derived growth-factor receptor inhibitor, suppresses lung cancer cell proliferation and migration. Onco Targets Ther. 2014;7:1215–1221. doi: 10.2147/OTT.S62946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takagi S, Takemoto A, Takami M, Oh-Hara T, Fujita N. Platelets promote osteosarcoma cell growth through activation of the platelet-derived growth factor receptor-Akt signaling axis. Cancer Sci. 2014;105(8):983–988. doi: 10.1111/cas.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Mandal D, Wang S, et al. Platelet-derived growth factor receptor beta inhibition increases tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitivity: imatinib and TRAIL dual therapy. Cancer. 2010;116(16):3892–3902. doi: 10.1002/cncr.25107. [DOI] [PubMed] [Google Scholar]
- 32.Geng ZM, Jha RK, Li B, et al. Sorafenib inhibition of hepatic stellate cell proliferation in tumor microenvironment of hepatocellular carcinoma: a study of the sorafenib mechanisms. Cell Biochem Biophys. 2014;69(3):717–724. doi: 10.1007/s12013-014-9858-y. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, Wang R, Yang Q, et al. Chemoresistance to gemcitabine in hepatoma cells induces epithelial-mesenchymal transition and involves activation of PDGF-D pathway. Oncotarget. 2013;4(11):1999–2009. doi: 10.18632/oncotarget.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizvi S, Mertens JC, Bronk SF, et al. Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis. J Biol Chem. 2014;289(33):22835–22849. doi: 10.1074/jbc.M114.563064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadamuro M, Nardo G, Indraccolo S, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58(3):1042–1053. doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Y, Guo JJ, Liu YM, et al. microRNA-34a inhibits the growth, invasion and metastasis of gastric cancer by targeting PDGFR and MET expression. Biosci Rep. 2014;34(3):pii: e00112. doi: 10.1042/BSR20140020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Garofalo M, Jeon YJ, Nuovo GJ, et al. MiR-34a/c-dependent PDGFR-α/β downregulation inhibits tumorigenesis and enhances TRAIL-induced apoptosis in lung cancer. PLoS One. 2013;8(6):e67581. doi: 10.1371/journal.pone.0067581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silber J, Jacobsen A, Ozawa T, et al. miR-34a repression in proneural malignant gliomas upregulates expression of its target PDGFRA and promotes tumorigenesis. PLoS one. 2012;7(3):e33844. doi: 10.1371/journal.pone.0033844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Põlajeva J, Swartling FJ, Jiang Y, et al. miRNA-21 is developmentally regulated in mouse brain and is co-expressed with SOX2 in glioma. BMC Cancer. 2012;12:378. doi: 10.1186/1471-2407-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazaki H, Yoshimatsu Y, Akatsu Y, et al. Expression of platelet-derived growth factor receptor β is maintained by prox1 in lymphatic endothelial cells and is required for tumor lymphangiogenesis. Cancer Sci. 2014;105(9):1116–1123. doi: 10.1111/cas.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laimer D, Dolznig H, Kollmann K, et al. PDGFR blockade is a rational and effective therapy for NPM-ALK-driven lymphomas. Nat Med. 2012;18(11):1699–1704. doi: 10.1038/nm.2966. [DOI] [PubMed] [Google Scholar]
- 42.Berghoff AS, Birner P, Streubel B, Kenner L, Preusser M. ALK gene aberrations and the JUN/JUNB/PDGFR axis in metastatic NSCLC. APMIS. 2014;122(9):867–872. doi: 10.1111/apm.12249. [DOI] [PubMed] [Google Scholar]
- 43.Akhavan D, Pourzia AL, Nourian AA, et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013a;3(5):534–547. doi: 10.1158/2159-8290.CD-12-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akhavan D, Pourzia AL, Nourian AA, et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013b;3(5):534–547. doi: 10.1158/2159-8290.CD-12-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin Y, Ding K, Li H, et al. Ponatinib efficiently kills imatinib-resistant chronic eosinophilic leukemia cells harboring gatekeeper mutant T674I FIP1L1-PDGFRα: roles of Mcl-1 and β-catenin. Mol Cancer. 2014;13:17. doi: 10.1186/1476-4598-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y, Shi X, Pan J. The conformational control inhibitor of tyrosine kinases DCC-2036 is effective for imatinib-resistant cells expressing T674I FIP1L1-PDGFRα. PLoS One. 2013;8(8e73059) doi: 10.1371/journal.pone.0073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gosenca D, Kellert B, Metzgeroth G, et al. Identification and functional characterization of imatinib-sensitive DTD1-PDGFRB and CCDC88C-PDGFRB fusion genes in eosinophilia-associated myeloid/lymphoid neoplasms. Genes Chromosomes Cancer. 2014;53(5):411–421. doi: 10.1002/gcc.22153. [DOI] [PubMed] [Google Scholar]
- 48.Iwasaki J, Kondo T, Darmanin S, et al. FIP1L1 presence in FIP1L1-RARA or FIP1L1-PDGFRA differentially contributes to the pathogenesis of distinct types of leukemia. Ann Hematol. 2014;93(9):1473–1481. doi: 10.1007/s00277-014-2085-1. [DOI] [PubMed] [Google Scholar]
- 49.Gorantla SP, Zirlik K, Reiter A, et al. F604S exchange in FIP1L1- PDGFRA enhances FIP1L1-PDGFRA protein stability via SHP-2 and SRC – a novel mode of kinase inhibitor resistance. Leukemia. 2015 doi: 10.1038/leu.2015.70. [DOI] [PubMed] [Google Scholar]
- 50.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. doi: 10.1155/2014/638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohishi J, Aoki M, Nabeshima K, et al. Imatinib mesylate inhibits cell growth of malignant peripheral nerve sheath tumors in vitro and in vivo through suppression of PDGFR-β. BMC Cancer. 2013;13:224. doi: 10.1186/1471-2407-13-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juliachs M, Muñoz C, Moutinho CA, et al. The PDGFRβ-AKT pathway contributes to CDDP-acquired resistance in testicular germ cell tumors. Clin Cancer Res. 2014;20(3):658–667. doi: 10.1158/1078-0432.CCR-13-1131. [DOI] [PubMed] [Google Scholar]
- 53.Ehnman M, Missiaglia E, Folestad E, et al. Distinct effects of ligand-induced PDGFRα and PDGFRβ signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res. 2013;73(7):2139–2149. doi: 10.1158/0008-5472.CAN-12-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamdan R, Zhou Z, Kleinerman ES. Blocking SDF-1α/CXCR4 downregulates PDGF-B and inhibits bone marrow-derived pericyte differentiation and tumor vascular expansion in Ewing tumors. Mol Cancer Ther. 2014;13(2):483–491. doi: 10.1158/1535-7163.MCT-13-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuge R, Kitadai Y, Shinagawa K, et al. mTOR and PDGF pathway blockade inhibits liver metastasis of colorectal cancer by modulating the tumor microenvironment. Am J Pathol. 2015;185(2):399–408. doi: 10.1016/j.ajpath.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Hara Y, Yamashita T, Oishi N, et al. TSU-68 ameliorates hepatocellular carcinoma growth by inhibiting microenvironmental platelet-derived growth factor signaling. Anticancer Res. 2015;35(3):1423–1431. [PubMed] [Google Scholar]
- 57.Wang W, Qi L, Tan M, et al. Effect of platelet-derived growth factor-B on renal cell carcinoma growth and progression. Urol Oncol. 2015;pii: S1078-1439(14):00468-2. doi: 10.1016/j.urolonc.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 58.Ho AL, Vasudeva SD, Laé M, et al. PDGF receptor alpha is an alternative mediator of rapamycin-induced Akt activation: implications for combination targeted therapy of synovial sarcoma. Cancer Res. 2012;72(17):4515–4525. doi: 10.1158/0008-5472.CAN-12-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheah CY, Burbury K, Apperley JF, et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood. 2014;123(23):3574–3577. doi: 10.1182/blood-2014-02-555607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chee CE, Krishnamurthi S, Nock CJ, et al. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist. 2013;18(10):1091–1092. doi: 10.1634/theoncologist.2013-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bible KC, Suman VJ, Molina JR, et al. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J Clin Endocrinol Metab. 2014;99(5):1687–1693. doi: 10.1210/jc.2013-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganjoo KN, Villalobos VM, Kamaya A, et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol. 2014;25(1):236–240. doi: 10.1093/annonc/mdt484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Angevin E, Lopez-Martin JA, Lin CC, et al. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013;19(5):1257–1268. doi: 10.1158/1078-0432.CCR-12-2885. [DOI] [PubMed] [Google Scholar]
- 64.Chiorean EG, Sweeney C, Youssoufian H, et al. A phase I study of olaratumab, an anti-platelet-derived growth factor receptor alpha (PDGFRα) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73(3):595–604. doi: 10.1007/s00280-014-2389-9. [DOI] [PubMed] [Google Scholar]
- 65.Basu B, Dean E, Puglisi M. First-in-human pharmacokinetic and pharmacodynamic study of the dual m-TORC 1/2 inhibitor, AZD2014. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubió-Casadevall J, Borràs J, Carmona-García MC, et al. Correlation between mutational status and survival and second cancer risk assessment in patients with gastrointestinal stromal tumors: a population-based study. World J Surg Oncol. 2015;13(1):474. doi: 10.1186/s12957-015-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]