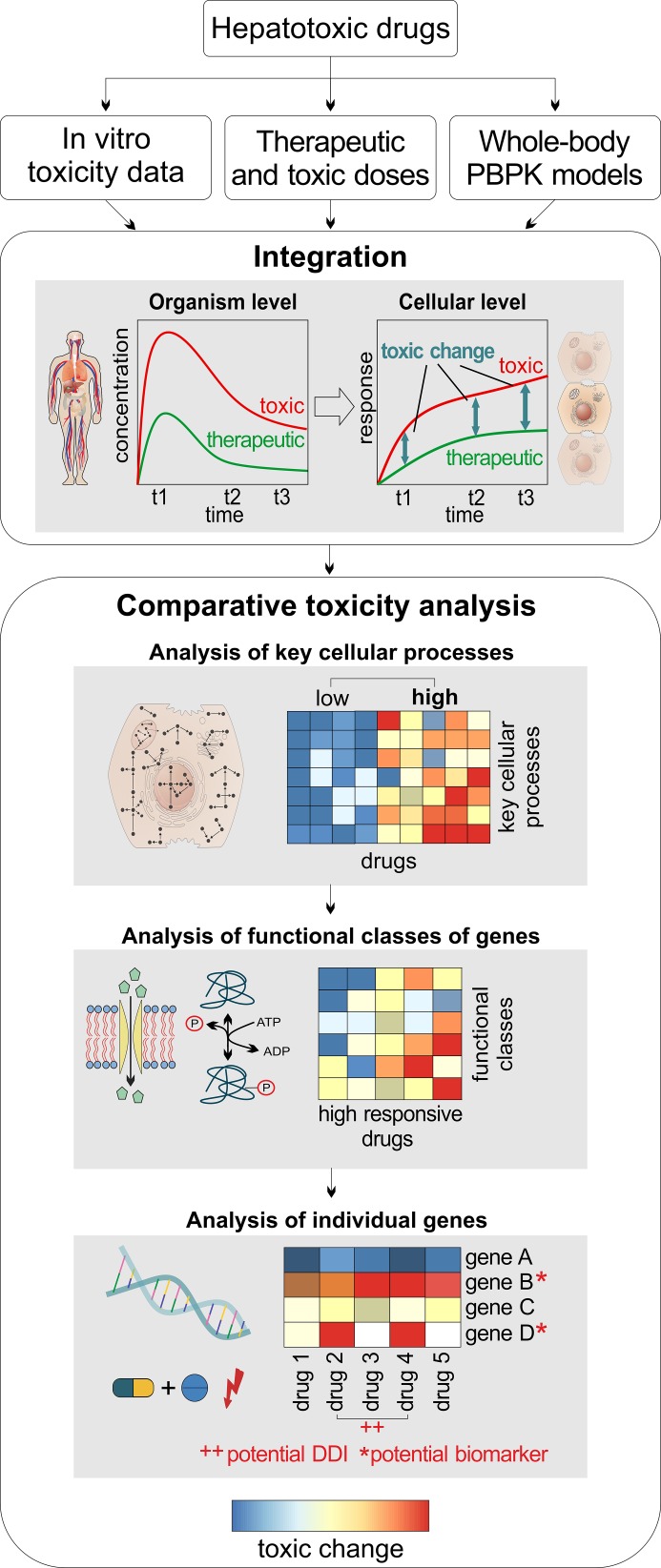
Fig 2. General workflow.
For a set of hepatotoxic drugs, in vitro toxicity data from Open TG-GATEs [21] were analyzed, therapeutic and toxic doses were identified in the literature, and whole-body PBPK models were developed and validated. Toxic changes were then predicted at different timepoints (2 h, 8 h, 24 h) by comparing cellular response following drug administration of therapeutic and toxic doses and were subsequently evaluated with regard to key cellular processes, functional classes of genes, and individual genes, respectively. (Illustration of cells and parts of human body adapted with permission [75], https://creativecommons.org/licenses/by/4.0/)