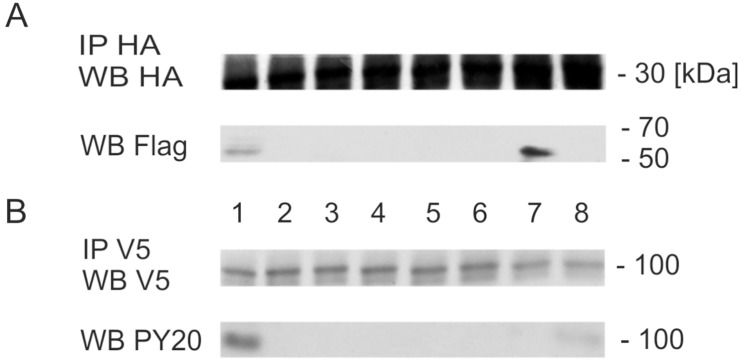
Fig 4. SmNck2 is part of the complex, and SmVKR1-phosphorylation occurs only when all complex-members are expressed.
Western blot analysis of complex members expressed in Xenopus oocytes. (A) following transfection with SmVKR1, Smβ-Int1, SmILK, SmPINCH, and SmNck2 constructs (see below) coimmunoprecipitation with anti-HA and subsequent Western blot (WB) analyses with anti-HA (HA-tagged: Smβ-Int1) and anti-Flag (SmNck2ΔSH3) confirmed the presence of Nck2 in the complex. (B) following coimmunoprecipitation with anti-V5, Western blot (WB) analyses with anti-V5 (V5-tagged SmVKR1) and anti-PY20 confirmed tyrosine phosphorylation of SmVKR1 only in case of a combination with Smβ-Int1, SmILK, SmPINCH as well as SmNck2 and without ligand (L-Arg) addition (lane 1, WB PY20). When deletion mutants of SmILK (SmILKΔAnk1; lane 2), SmPINCH (SmPINCHΔLIM1, SmPINCHΔLIM4; lanes 3, 4), SmNck2 (SmNck2ΔSH3; lane 5), SmVKR1 (dk, dead kinase mutant; [22]; lane 6), or the ILK inhibitor QLT-0267 (1 μM; lane 7) were used, no tyrosine phosphorylation of SmVKR1 was detected. As a positive control, L-Arg was added as ligand, which induced SmVKR1 tyrosine phosphorylation as expected (lane 8).