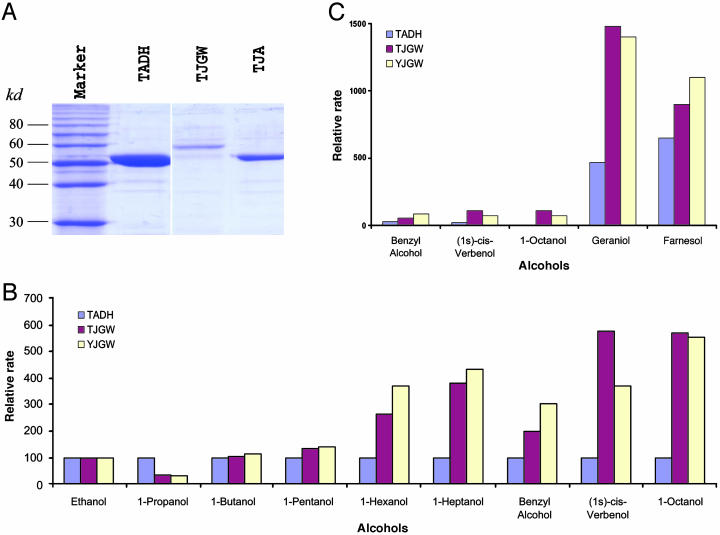
Fig. 3.
Purification of JGW and its enzymatic properties. (A) Purification of JGW and ADH revealed by Coomassie blue staining of SDS/PAGE gels. TADH, ADH of D. teissieri; TJA, ADH-derived domain in JGW of D. teissieri; TJGW, JGW of D. teissieri. (B) The changes of Vmax, relative to ethanol, which is the abundant natural substrate of ancestral ADH, of JGW toward short- and medium-chain alcohols measured according to the method of Waller (35) in three proteins: ADH of D. teissieri (TADH, blue), JGW of D. teissieri (TJGW, red), and JGW of D. yakuba (YJGW, yellow) (these abbreviations and colors are the same in C). Substrates shown are a series of primary alcohols, including benzyl alcohol. (C) The changes of Vmax, relative to ethanol, of JGW toward long-chain alcohols (see Materials and Methods). As we explained in Materials and Methods, with variation in activity among replicate assays <5% of the average rates between repeats of experiments, the variation in the rates between enzymes is statistically significant. We observed that the differences between JGW and ADH in the activities shown in this figure for long-chain alcohol substrates (B, 1-hexanol, 1-heptanol, benzyl alcohol, (1S)-cis-verbenol, and 1-octanol; C, geraniol and farnesol) are at least 7 times greater than standard deviation, indicating that these differences between JGW and ADH are statistically significant (P < 0.001).