Abstract
High mobility group box 1 (HMGB1) is a pivotal mediator of sepsis progression. Remifentanil, an opioid agonist, has demonstrated anti-inflammatory effects in septic mice. However, it is not yet known whether remifentanil affects the expression of HMGB1. We investigated the effects of remifentanil on HMGB1 expression and the underlying mechanism in septic rats. Forty-eight male Sprague-Dawley rats were randomly divided into 3 groups; a sham group, a cecal ligation and puncture (CLP) group, and a CLP with remifentanil treatment (Remi) group. The rat model of CLP was used to examine plasma concentrations of proinflammatory cytokines, tissue HMGB1 mRNA and the activity of nuclear factor (NF)-κB in the liver, lungs, kidneys, and ileum. Pathologic changes and immunohistochemical staining of NF-κB in the liver, lungs, and kidneys tissue were observed. We found that remifentanil treatment suppressed the level of serum interleukin (IL)-6 and tumor necrosis factor (TNF)-α 6 hours after CLP, and serum HMGB1 24 hours after CLP. HMGB1 mRNA levels and the activity of NF-κB in multiple organs decreased by remifentanil treatment 24 hours after CLP. Remifentanil treatment also attenuated nuclear expression of NF-κB in immunohistochemical staining and mitigated pathologic changes in multiple organs. Altogether, these results suggested that remifentanil inhibited expression of HMGB1 in vital organs and release of HMGB1 into plasma. The mechanism was related to the inhibitory effect of remifentanil on the release of proinflammatory cytokines and activation of NF-κB.
Keywords: HMGB1 Protein, Inflammation, NF-κB, Remifentanil, Sepsis
INTRODUCTION
Sepsis is an uncontrolled systemic inflammatory response that can result in multiple organ dysfunction and death. An imbalance between the proinflammatory response and the anti-inflammatory response is the primary cause of a poor prognosis in sepsis (1). Overwhelming systemic inflammatory responses can lead to lethal multiple organ damages. Accordingly, many studies have been carried out to elucidate the function of inflammatory cytokines and investigate strategies to attenuate the excessive inflammatory responses in order to improve prognosis. However, the effects of these strategies are still controversial and require further studies.
High mobility group box 1 (HMGB1) is a nonhistone chromosomal protein that stabilizes nucleosome formation and facilitates transcription (2). HMGB1 also acts as a principle inflammatory mediator in sepsis and plays an important role in sepsis progression following its release from innate immune cells and necrotic cells (3). Many studies have indicated a positive correlation between HMGB1 levels and the severity of sepsis in patients and experimental animals (4,5,6). Inhibition of HMGB1 by antibodies or inhibitors has been shown to decrease the lethality of sepsis in mice (7,8). Therefore, inhibition of HMGB1 expression has been investigated as a target for the treatment of sepsis.
Remifentanil, a synthetic opioid agonist, is used as a sedative or anesthesia adjuvant in critically ill or septic patients because it reduces the requirement for other anesthetics while maintaining cardiovascular performance. Several recent studies have reported that remifentanil has anti-inflammatory effects in vitro and in vivo (9,10). Remifentanil inhibits proinflammatory cytokines production and suppresses inducible nitric oxide synthase (iNOS) expression in a murine cecal ligation and puncture (CLP) model (11). In addition, remifentanil has a protective effect in lipopolysaccharide (LPS)-induced acute lung injury in rats by down regulating the nuclear factor (NF)-κB signaling pathway and acute-phase inflammatory cytokines (12). Although the relationship between early proinflammatory cytokines, NF-κB pathway, and HMGB1 has been elucidated (2), the effect of remifentanil on HMGB1 expression in sepsis has not been explored.
Therefore, we aimed to determine whether remifentanil attenuated the expression and release of HMGB1, a crucial mediator of sepsis progression, in CLP-induced septic rats and to investigate the underlying mechanism.
MATERIALS AND METHODS
Animals and materials
All experimental protocols were approved by the Experimental Animal Ethics Committee of the Catholic University of Korea, St. Vincent's Hospital. The care and handling of the animals were in accordance with the criteria outlined in the guide for the care and use of laboratory animals prepared by the National Institutes of Health (NIH).
Adult (8-week-old) male Sprague-Dawley rats weighing 250–280 g were obtained from the experimental animal center of the Catholic University St. Vincent's Hospital. The rats were housed 3 per cage in the regular animal room and given standard laboratory food and ultraviolet (UV) sterilized tap water ad libitum. Ultiva® (remifentanil hydrochloride 1.1 mg/vial; GlaxoSmithKline PLC., Brentford, United Kingdom) was purchased from Daesung Wholesale Pharmacy after approval of the Kyong-in Food and Drugs Administration (Batch No. Kyongin 313).
Groups and experimental protocols
Forty-eight rats were divided into 3 groups as follows: sham (n = 16), CLP (n = 16), and CLP with remifentanil treatment (Remi) (n = 16) groups. Sepsis was induced in the CLP and Remi groups by CLP as previously described (13). The procedure was carried out at the same time of the day for all rats to account for the effect of the circadian rhythm on the inflammatory response. After fasting for 8 hours, the rats were anesthetized with 2%–3% isoflurane with 50% oxygen. Tail veins were cannulated with a 24-gauge angio-catheter, then connected to a micro-injection pump. A 2-cm midline incision was made on the anterior abdomen and the cecum was exposed and ligated below the ileocecal junction without causing bowel obstruction. The cecum was punctured twice with an 18-gauge needle and a small amount of cecal content was squeezed through punctures. The cecum was placed back and the peritoneal wall and skin incisions were closed. Rats in the sham group underwent a similar surgery, but without the cecal ligation or puncture. All animals received 3 mL of warm sterile normal saline intraperitoneally and 0.2 mL of 0.5% bupivacaine injection at the incision site. For postoperative pain control, all animals were injected with buprenorphine (0.1 mg/kg subcutaneously) immediately after surgery. Rats in the Remi group were administered an intravenous injection of remifentanil (0.04 mg/kg) for 40 minutes immediately after the surgery. In the sham and CLP groups, normal saline replaced remifentanil. All rats were kept warm until they recovered from anesthesia, after which they were returned to their cages. No antibiotics were administered.
Organ tissue preparation
Eight rats from each group were sacrificed with 150 mg/kg of sodium pentobarbital intraperitoneally 6 or 24 hours after CLP. Blood samples were harvested from the inferior vena cava (3 mL from each) before death. The samples were centrifuged for 30 minutes at 4°C, and 3,000 rpm, and then stored at −80°C for further study. Tissue specimens were collected immediately after death. Tissues from the liver, kidneys, lungs, and ileum (5 cm proximal to the ileocecal valve) were separately homogenized for real-time polymerase chain reaction (PCR) analysis or western blot analysis. For histologic examinations and immunohistochemistry, rats were perfused through the left cardiac ventricle with 0.9% sodium chloride followed by 4% paraformaldehyde. The liver, lungs, and kidneys were removed after they became stiff and were placed in a matrix. Tissues were sliced to 2 mm thickness and immersed overnight in 4% paraformaldehyde. Each slice was processed in paraffin wax and cut into 5-μm thick sections.
Measurements of alanine aminotransferase (ALT) and creatinine
Serum ALT and creatinine were measured using an automatic biochemistry analyzer (Hitachi7180; Hitachi High-Technologies Corp., Tokyo, Japan) with commercially available clinical assay kits.
Measurements of cytokine levels in serum by enzyme linked immunosorbent assay (ELISA)
Serum was stored at −80°C after centrifugation, as described above. Serum HMGB1 levels were determined using a rat HMGB1 ELISA Kit (IBL-Hamburg, Hamburg, Germany) according to the instructions from the manufacturer. Serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels were determined using a rat TNF-α Quantikine ELISA Kit (R & D Systems, Minneapolis, MN, USA) and a rat IL-6 Quantikine ELISA Kit (R & D Systems) respectively.
Real-time PCR analysis to determine the expression of HMGB1 mRNA
Total RNA was extracted from tissues of the lung, liver, and kidney using an RNeasy Plus Mini Kit (QIAGEN GmbH, Hilden, Germany). RNA was reverse transcribed using a PrimeScript RT Reagent Kit (TaKaRa Bio Inc., Shiga, Japan). Relative expression levels of mRNA were determined using a Roche Diagnostics LightCycler 2.0 Real-Time PCR System (Roche Diagnostics GmbH, Mannheim, Germany) with a SensiFAST™ SYBR® Hi-ROX Kit (Bioline, Luckenwalde, Germany) and gene-specific primers (Table 1). A total volume of 10 μL of reaction system liquid was subjected to the following PCR program: 1 cycle at 95°C for 30 seconds (initial denaturation), followed by 41 cycles at 95°C for 5 seconds, 60°C for 25 seconds. All protocols were performed according to the manufacturer's instructions.
Table 1. Primer sequences for real-time PCR.
| Primers | Accession No. | Sequences (5' → 3') | Size, bp | Cycle No. | Annealing temperature, ℃ |
|---|---|---|---|---|---|
| HMGB1 | NM012963 | F: GGCGAGCATCCTGGCTTATC | 142 | 35 | 60 |
| R: AGGCAGCAATATCCTTCTCATAC | |||||
| GAPDH | NM0170084 | F: GCACAGTCAAGGCTGAGAATG | 142 | 35 | 60 |
| R: ATGGTGGTGAAGACGCCAGTA |
PCR = polymerase chain reaction, HMGB1 = high mobility group box1, GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
Western blotting analysis for activity of NF-κB
Fresh tissues from the liver, lungs, kidneys, and ileum were homogenized and lysates were made using the PRO-PREP protein extraction solution (Intron Biotech, Sungnam, Korea). Lysates were clarified by centrifugation at 13,000 rpm at 4°C for 5 minutes and the protein concentration of the supernatant was determined with a Bradford assay kit (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein extract were loaded on 10% polyacrylamide gels and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The extracts were then transferred to polyvinylidene difluoride membranes using Semi-Dry Trans-Blot Cells (Bio-Rad Laboratories, Hercules, CA, USA). Polyvinylidene difluoride membranes containing transferred proteins were blocked with tris-buffered saline (TBS) containing 0.1% Tween 20 (TBST) and 5% skim milk for 1 hour at 25°C. After 3 washes with TBST, membranes were incubated with rabbit anti NF-κB (p65) antibody (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and, anti β-actin antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. After another 3 washes with TBST, membranes were incubated with horseradish peroxidase linked secondary antibodies (1:1,000; Cell Signaling Technology) for 1 hour at 25°C. After 3 final washes in TBST, membranes were treated with electrochemiluminescence reagents (Amersham, Buckinghamshire, UK), and then exposed digitally with an Image Reader ImageQuant™ LAS 4000 mini (GE Healthcare Europe GmbH, Freiburg, Germany). Proteins were quantified for statistical analysis using Multi Gauge version 3.0 software (Fujifilm Life Science, Tokyo, Japan).
Immunohistochemistry for NF-κB
Paraffin section were dried for 45 minutes, and then dewaxed in 2 changes of xylene for 15 and 20 minutes each, followed by a descending ethanol series and antigen retrieval in citric acid. The sections were incubated in 3% hydrogen peroxide for 15 minutes in a humidistat box at 25°C and rinsed 3 times in phosphate buffered saline (PBS) for 5 minutes. After an overnight incubation at 4°C with monoclonal rabbit anti rat NF-κB antibody (1:400 for kidney and lung, 1:1,000 for liver; Cell Signaling Technology), sections were incubated with ImmPRESS Anti-Rabbit Ig secondary antibodies (Ready to use, VECTOR Laboratories, Burlingame, CA, USA) at 25°C for 1 hour. After being washed 3 times with PBS for 5 minutes each, the sections were developed with 3,3'-diaminobenzidine (DAB) peroxidase substrate (VECTOR Laboratories), terminated in distilled water, and counterstained with hematoxylin for 5 minutes. The slides were then dehydrated in graded concentrations of ethanol, cleared in 2 changes of xylene for 10 minutes each, and mounted with Canada balsam. The sections were examined and photographed with an Olympus BX51 light microscope (Olympus, Tokyo, Japan) at × 400. At least 4 sections from each organ were evaluated.
Histopathology (hematoxylin and eosin [H & E] stain)
Paraffin sections were dried for 45 minutes, and then dewaxed twice in 2 changes of xylene for 15 and 20 minutes each, followed by a descending ethanol series and a rinse in running tap water for 2 minutes. Thereafter, the slides were stained with hematoxylin for 5 minutes, washed in tap water, differentiated in 1% acid alcohol, blued in 1% ammonia water, and counterstained with eosin for 1 minute. The slides were then washed in running tap water, dehydrated by a descending ethanol series, cleared in 2 changes of xylene for 10 minutes each, and mounted with neutral gum. The slides were examined and photographed by an Olympus BX51 light microscope (Olympus) to evaluate inflammation and tissue damage.
Statistical analysis
Statistical analysis was carried out using SPSS® statistical software, version 21.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Continuous variables were tested for normal distribution using the Kolmogorov-Smirnoff test. Data are presented as mean ± standard deviation (SD). Statistical analysis was performed using 1-way analysis of variance (ANOVA) followed by Bonferroni or Turkey post hoc test for normally distributed data or Kruskal-Wallis test with Mann-Whitney-U post hoc test, as appropriate. For comparison of discrete variables, the Mann-Whitney test was used after the Kruskal-Wallis test. A value of P < 0.05 was considered significant.
Ethics statement
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) in St. Vincent's Hospital, The Catholic University of Korea (animal IRB 15-20).
RESULTS
General status
During intravenous infusion of the study drug, all rats maintained self-respiration. In Remi group, rats showed slowed respiratory rate during infusion of remifentail, but distress symptoms such as gasping through mouth, blue extremities and apnea did not occur. Approximately 20 minutes after the end of remifentanil infusion, all rats recovered from the anesthesia, could move actively.
With the progression of sepsis, sluggish actions, diarrhea, lethargy, and decreased water and food intake occurred in rats of the CLP group. The rats in the Remi group also appeared less active and piloerection but their symptoms were less severe than rats in the CLP group.
ALT and creatinine
To investigate the effect of remifentanil on CLP-induced organ injury, serum ALT and creatinine levels were determined. ALT was significantly higher 6 hours after surgery in the CLP group compared to sham group (P = 0.032, Table 2). However, there were no significant differences between ALT in Remi group and Sham group 6 hours after surgery (P = 0.101). ALT was significantly higher 24 hours after surgery in the CLP and Remi groups than in the sham group (P = 0.040 and 0.026, respectively) but ALT in the Remi group was significantly lower than that in the CLP group (P = 0.016, Table 2). There were no significant differences between groups in creatinine levels 6 hours or 24 hours after surgery (Table 2).
Table 2. Temporal changes in serum ALT and creatinine concentration after underwent CLP/sham operation in each group of rats (n = 8).
| Groups | ALT, U/L | Creatinine, mg/dL | ||
|---|---|---|---|---|
| 6 hr | 24 hr | 6 hr | 24 hr | |
| Sham | 43.64 ± 3.44† | 44.96 ± 3.14† | 0.36 ± 0.06 | 0.42 ± 0.03 |
| CLP | 97.52 ± 28.38* | 173.50 ± 84.20* | 0.40 ± 0.02 | 0.51 ± 0.13 |
| Remi | 60.74 ± 13.05† | 70.28 ± 12.90*,† | 0.37 ± 0.05 | 0.45 ± 0.02 |
Data were expressed as the mean ± SD.
ALT = alanine aminotransferase, CLP = cecal ligation and puncture, Sham = sham operation group, Remi = cecal ligation and puncture with remifentanil treatment, SD = standard deviation.
*P < 0.05 compared with sham group; †P < 0.05 compared with CLP group.
Plasma cytokine levels (TNF-α, IL-6, and HMGB1)
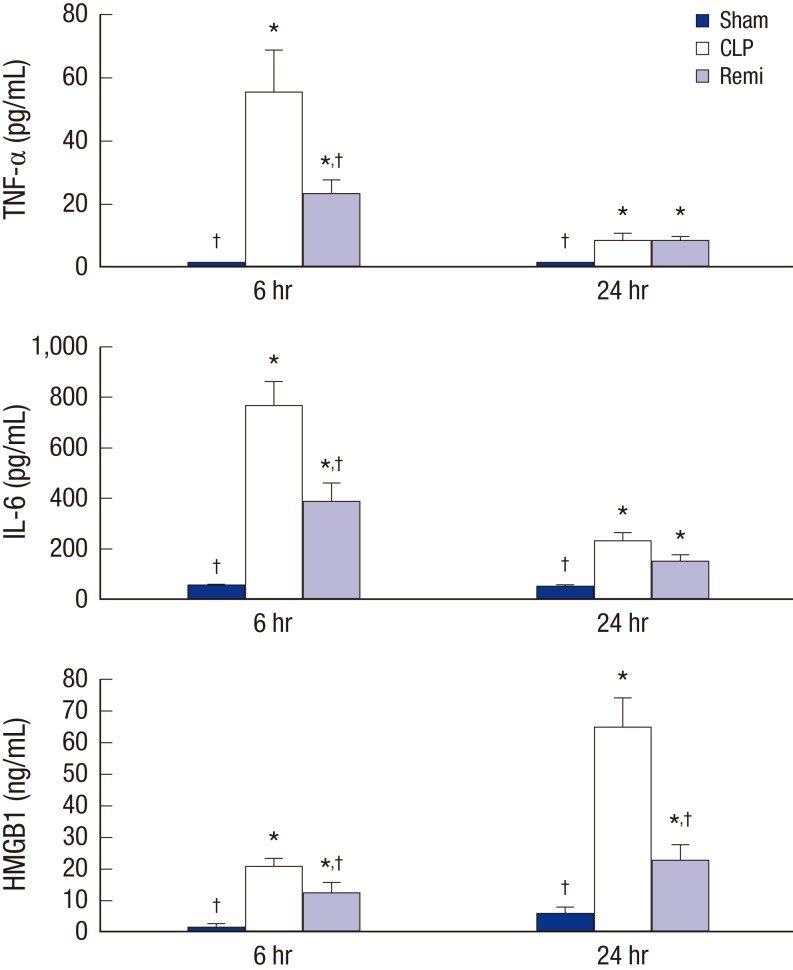
To evaluate the effect of remifentanil on early onset proinflammatory cytokines in CLP-induced septic rats, serum samples were collected to evaluate TNF-α and IL-6 levels using ELISA. TNF-α levels were significantly higher 6 hours after surgery in the CLP group than in the sham group (P = 0.026). TNF-α levels in the Remi group were significantly higher than in the sham group (P = 0.011), but significantly lower than in the CLP group (P = 0.020) 6 hours after surgery. TNF-α levels 24 hours after surgery in the CLP and Remi groups were significantly higher than in the sham group (P = 0.003 and 0.003, respectively; Fig. 1).
Fig. 1.
Effects of remifentanil treatment in inflammatory cytokines release 6 and 24 hours after sham/CLP operation. Serum TNF-α, IL-6, and HMGB1 concentration were analyzed by ELISA in rats subjected to sham/CLP operation. Data were presented as mean ± SD (n = 8).
TNF = tumor necrosis factor, IL = interleukin, HMGB1 = high mobility group box 1, ELISA = enzyme linked immunosorbent assay, SD = standard deviation, Sham = sham operation group, CLP = cecal ligation and puncture with normal saline group, Remi = cecal ligation and puncture with remifentanil treatment group.
*P < 0.05 vs. sham; †P < 0.05 vs. CLP.
Differences in IL-6 levels were similar to differences in TNF-α levels. A significant increase in IL-6 was observed 6 hours after surgery in the CLP group compared to the sham group (P = 0.002), but the IL-6 level in the Remi group was significantly lower than in the CLP group (P = 0.019, Fig. 1).
Serum HMGB1 levels were also evaluated by ELISA to determine the effect of remifentanil on systemic HMGB1 release in CLP-induced septic rats. As shown in Fig. 1, the HMGB1 levels 6 hours after surgery in the CLP and Remi group were significantly higher than in the sham group (P = 0.010 and 0.034, respectively). In addition, HMGB1 levels in the Remi group were significantly lower than those in the CLP group (P = 0.008). HMGB1 levels 24 hours after surgery in the Remi group were significantly higher than those in the sham group (P = 0.042), but significantly lower than those in the CLP group (P = 0.010).
Real-time PCR (HMGB1 mRNA expression)
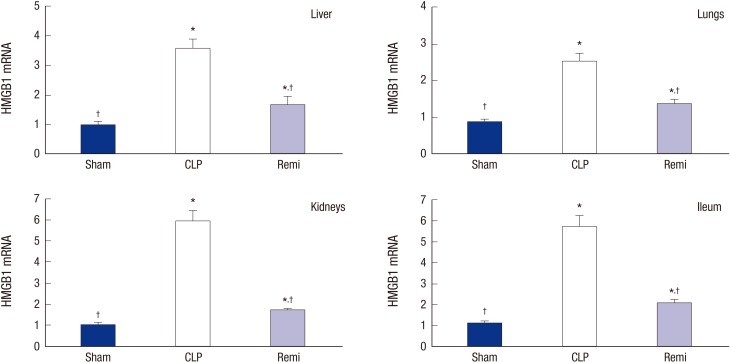
To further explore the effect of remifentanil on HMGB1 transcription in various organs, we extracted total RNA from the liver, lungs, kidneys, and ileum after CLP. HMGB1 mRNA levels were determined by real-time PCR. As shown in Fig. 2, HMGB1 mRNA levels in the organs of rats in the CLP group significantly increased than those in the sham 24 hours after surgery (P = 0.001 for liver, P = 0.002 for lungs, P = 0.002 for kidneys, and P = 0.001 for ileum). HMGB1 mRNA levels in the Remi group were significantly lower than those in the CLP group 24 hours after CLP (P = 0.003 for liver, P = 0.004 for lungs, P = 0.002 for kidneys, and P = 0.002 for ileum).
Fig. 2.
The expressions of HMGB1 mRNA in tissues of rats in each group 24 hours after sham/CLP operation. Liver, lungs, kidneys, and ileum were collected 24 hours after surgery. HMGB1 mRNA expression in multiple organs were measured by real-time PCR. Data were presented as mean ± SD (n = 8).
HMGB1 = high mobility group box 1, PCR = polymerase chain reaction, SD = standard deviation, Sham = sham operation group, CLP = cecal ligation and puncture with normal saline group, Remi = cecal ligation and puncture with remifentanil treatment group.
*P < 0.05 vs. sham; †P < 0.05 vs. CLP.
Western blot analysis of NF-κB
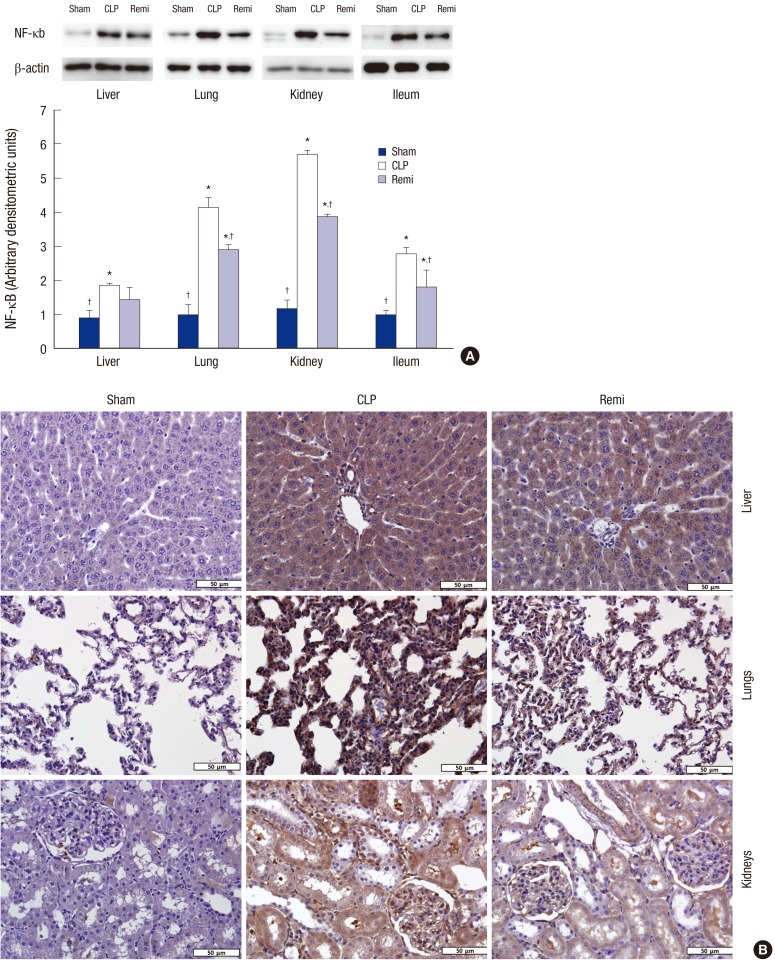
The effect of remifentanil on NF-κB activation was examined to investigate the anti-inflammatory mechanisms of remifentanil 24 hours after surgery. Fig. 3A indicates that the expression of NF-κB activation, which was increased in the CLP group compared to that of the sham group (P = 0.037 for liver, P < 0.001 for lungs, P < 0.001 for kidneys, and P = 0.001 for ileum). The expression of NF-κB activation in the Remi group was decreased significantly compared to that of the CLP group (P = 0.035 for lungs, P = 0.011 for kidneys, and P = 0.027 for ileum).
Fig. 3.
Effects of remifentanil treatment on activity of NF-κB in organs of septic rats 24 hours after CLP operation. (A) Liver, Lungs, kidneys, and ileum were collected 24 hours after sham/CLP operation and NF-κB p65 activation was analyzed by Western blot. Data were presented as mean ± SD (n = 8). (B) Immunohistochemical staining of NF-κB p65 in liver, lungs, and kidneys from rats subjected to sham/CLP operation 24 hours after surgery. Original magnification: × 400.
NF = nuclear factor, SD = standard deviation, Sham = sham operation group, CLP = cecal ligation and puncture with normal saline group, Remi = cecal ligation and puncture with remifentanil treatment group.
*P < 0.05 vs. sham; †P < 0.05 vs. CLP.
Immunohistochemistry for NF-κB
Immunohistochemistry was performed to determine the nuclear fraction of NF-κB activity in tissues from the liver, lungs, and kidney. The immunohistochemical staining of NF-κB p65 in tissues from the sham group was mainly in the cytoplasm with light brown coloration (Fig. 3). On the other hand, the tissue staining of NF-κB p65 of rats subjected to CLP was dark brown and the nucleus could not be seen clearly due to the strong staining of NF-κB p65. However, in Remi group, tissue staining became lighter compared to CLP group and nucleus was blue and clearly seen. Therefore, remifentanil treatment inhibited nuclear translocation and activity of NF-κB in various organs of CLP-induced septic rats.
Histopathology (H & E stain)
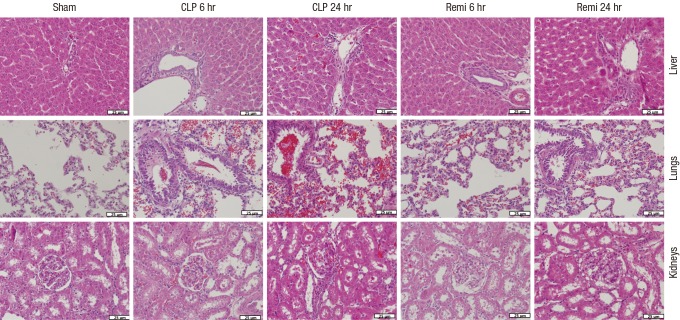
In the sham group, no pathological changes occurred, whereas many inflammatory cell infiltrations and severe injuries in multiple organs of the CLP group occurred 6 hours and 24 hours after surgery (Fig. 4). The major acute inflammatory injuries in the lungs from CLP-induced septic rats were infiltration of leukocytes and leakage of erythrocytes into alveolar and interstitial spaces, edema and thickening of the alveolar capillary membrane. Injuries in the liver included substantial hepatic tissue malformation, intracellular and interstitial edema, and necrosis. In the kidneys, the major morphological alterations were interstitial leukocyte infiltration, intercapillary cell proliferation, endothelial cell swelling, and kidney tubular hyperemia. However, organs from the Remi group showed minor damage and fewer inflammatory cell responses.
Fig. 4.
H & E stained sections of liver, lungs and kidneys from rats subjected to sham/CLP operation 6 hours and 24 hours after surgery. Original magnification: × 400.
H & E = hematoxylin and eosin, Sham = sham operation group, CLP = Cecal ligation and puncture with normal saline group, Remi = cecal ligation and puncture with remifentanil treatment group.
DISCUSSION
In the present study, the effects of remifentanil on inflammatory cytokines and organ damage were evaluated in a septic rat model. We observed that remifentanil had protective effects as evidenced by suppression of the expression and release of HMGB1 in CLP-induced septic rats. We also determined that remifentanil attenuated NF-κB activation.
The pathogenesis of sepsis can be explained by an early proinflammatory response, a failure of the compensatory anti-inflammatory response, and immunoparalysis (1). During the initial hyperinflammatory phase, major proinflammatory cytokines, including IL-1, IL-6, TNF-α, and chemokines, increase. Excessive proinflammatory cytokines facilitate inflammation by enhancing endothelial cell-leukocyte adhesion, promoting the release of nitric oxide and arachidonic acid metabolites, and activating the complement cascade. Several studies have evaluated the effects of unopposed proinflammatory cytokines in the outcome of sepsis and indicated that inhibition of the cytokines prevents an exaggerated inflammatory response and attenuates organ dysfunction (14,15). TNF-α is 1 of the initial mediators of sepsis progression and is, known to be closely correlated with mortality (16). High concentrations of IL-6 are associated with mortality and morbidity in septic patients (17). Early proinflammatory cytokines also activate innate immune cells to release late mediators of sepsis such as HMGB1. Thereafter, sepsis may progress to septic shock and multiple organ failure. Therefore, we investigated serum proinflammatory cytokines, including IL-6, TNF-α, and HMGB1, to explore the beneficial effects of remifentanil on CLP-induced sepsis.
The CLP-induced sepsis model resembles the progression of human sepsis in that there is an early hyperdynamic phase followed by a late hypodynamic phase (13). Considering the clinical manifestation of CLP and the results of the present study, the sepsis model was appropriate for the present study. In septic rats, CLP led to an increase in the serum concentrations of TNF-α and IL-6 in the acute phase and HMGB1 in the delayed phase. In contrast, administration of remifentanil attenuated the generation of these serum pro-inflammatory cytokines. Our results were similar to previous findings that intravenous anesthetics attenuated acute and delayed phase proinflammatory cytokines and led to improved outcomes (18,19).
HMGB1, a nonhistone chromosomal protein, is produced by nearly all cell types and mediates diverse cellular functions (3). When inflammation or injuries occur, HMGB1 is actively released by innate immune cells to induce inflammation, and is passively released by necrotic cells to conduct an injury signal to neighboring cells (3). Once released, HMGB1 itself signals through the receptor for advanced glycation end products (RAGE) and toll-like receptors (TLRs), TLR2 and TLR4. In addition, it activates the NF-κB signaling pathway and the mitogen-activated protein kinase (MAPK) pathway (20). As a result, innate immune cells are activated to release proinflammatory cytokines, endothelial adhesion molecules are upregulated, and chemotactic cell movement and epithelial cell dysfunctions occur, all of which sustain the inflammatory response (3). Therefore, HMGB1 plasma concentrations parallel the severity of sepsis (21) and onset of animal lethality from endotoxemia or septic shock. Wang et al. (7) reported that delayed administration of antibodies to HMGB1 attenuated endotoxin lethality in mice and administration of HMGB1 itself was lethal. Clinical studies also demonstrated that a high serum HMGB1 level was maintained in patients with severe sepsis and septic shock (6,21). Several experimental studies also substantiated that agents decreasing HMGB1 expression attenuates multiple organ failure and reduced mortality (22,23,24). Data from the present study indicated that increased serum HMGB1 and HMGB1 mRNA expression in the lungs, liver, ileum, and kidneys was correlated with histological findings and elevated levels of plasma ALT in septic rats. Remifentanil treatment substantially reduced serum HMGB1 levels and alleviated mRNA expression and inflammatory histologic alteration caused by sepsis in multiple organs. The results demonstrated that remifentanil down-regulated excessive HMGB1 gene expression secondary to septic change.
HMGB1 signaling is amplified at several levels by reciprocal functional relationships. NF-κB, mediated by TLR and RAGE signaling, plays a crucial role in sustaining HMGB1 signaling (2). NF-κB is a transcription factor that regulates many target genes in different cells to facilitate their various functions (25). HMGB1-induced RAGE can lead to the phosphorylation and degradation of IκB and NF-κB-mediated activation of gene expression, NF-κB signaling pathway also affect HMGB1 expression by cross-talk (2). When stimulated by bacterial products and viruses, the activation and nuclear translocation of NF-κB leads to increased transcription of genes encoding proinflammatory cytokines, including HMGB1, chemokines, immune receptors, and cell surface adhesion molecules, therefore, enhance leukocyte infiltration. NF-κB also mediates the upregulation of HMGB1 receptors (2). Accordingly, to elucidate the underlying mechanisms for the attenuation of HMGB1 in CLP-induced sepsis by remifentanil, we investigated NF-κB activation in vital organs. Our data showed that remifentanil inhibited both the NF-κB signaling pathway and the expression of HMGB1. This result was partially consistent with a recent study, which reported that remifentanil reduced the activation of NF-κB by inhibition of IκBα degradation and limited cytokine transcription in an LPS-induced lung injury model (12). Downregulation of NF-κB resulted in the attenuation of lung injury (12). Therefore, inhibitory effects of remifentanil on activity of NF-κB may also contribute to organ protection by preventing exaggerated inflammatory process of sepsis.
The anti-inflammatory effects of opioids, including remifentanil, have been widely studied, but the exact mechanism has not yet been elucidated. Opioids exert rapid effects on immune cells via active opioid receptors on the cell surface (26). During the delayed effect, activation of central opioid receptors affects the peripheral immune system via the stimulation of the sympathetic nervous system and its effects on lymphoid organs (27). Among various opioids, only remifentanil attenuates the activation of human neutrophils exposed to LPS by decreasing the activation of inflammatory cytokines and p38 MAPKs in vitro (28). Because RAGE, the cell surface receptor of HMGB1, can signal through the activation of p38 MAPK and lead to NF-κB-mediated activation of gene expression (2), the results of the present study can be explained by these mechanisms. Zongze et al. (11) obtained similar results that indicated remifentanil has protective effects in septic mice by repressing inflammatory cytokines and pathologic changes. In addition, several clinical studies supported the results from the present study. Ke et al. (29) found that total intravenous anesthesia using propofol and remifentanil attenuates TNF-α and IL-6 induction after open cholecystectomy. A few studies also demonstrated that continuous infusion of remifentanil could suppress an exaggerated inflammatory response and endocrine stress reaction in patients undergoing cardiac surgery (30,31). Neuroendocrine response reduced by remifentanil during operation could also affect the postoperative immune function that may result in good prognosis such as shorter intensive care unit (ICU) stay (30). However, the precise mechanisms by which remifentanil attenuates the expression of HMGB1 should be further studied. The effects of remifentanil on related upstream signaling pathways, including RAGE, TLRs, and myeloid differentiation primary response 88 (MyD88), still need to be clarified.
Mortality in sepsis is crucially influenced by organ injury and dysfunction. One important consideration for the choice of drugs may be whether the drug has protective effects on vital organs. The effect of remifentanil in the function of the lungs was already evaluated in several studies. Zhang et al. (12) showed that remifentanil reduced myeloperoxidase (MPO) activity in rats with LPS-induced lung injury. Remifentanil also protected intestines by decreasing the percentage of damaged villi and the amount of mucosal damage in an intestinal ischemia-reperfusion injury model (32). Therefore, we evaluated mRNA levels in the vital organs, serum ALT, and creatinine. The liver contains the largest number of macrophages (Kupffer cells) in the body that can initiate the systemic inflammatory response. Serum ALT would be elevated by increased membrane permeability caused by cell injury. Data from the present study implied that remifentanil had a protective effect on organ injury and attenuated the elevation of ALT. Serum creatinine reflects the function of the kidneys. Although the difference was not significant, the creatinine level of the remifentanil treatment group was lower than that in the CLP group. These results indicated a positive correlation between organ injury and HMGB1 mRNA expression in multiple organs.
Because the administration of anesthetics is inevitable in severely septic or critically ill patients, the anti-inflammatory effects of various anesthetics have been studied. Recent studies demonstrated that the administration of intravenous anesthetics or local anesthetics, such as ketamine, propofol, lidocaine, and levobupivacaine, inhibited HMGB1 release and manifested protective effects against sepsis (18,23,33,34). However, the cardiovascular depressive effects of these drugs tend to prevent their use in patients with sepsis. Remifentanil is frequently administered as an analgesic agent in patient undergoing general anesthesia in operating room or invasive care in ICU. Remifentanil is also proved to be a well-tolerated agent for analgesia-based sedation of critically ill patients in ICU for up to 10 days (35). Although patients with septic shock are usually hemodynamically unstable, use of remifentanil may be preferable because it allows for fast discontinuation in the event of hemodynamic instability (36). In the present study, we deduced that remifentanil could be helpful by decreasing the exaggerated inflammatory response during anesthesia and sedation of septic patients. Therefore, remifentanil may be advantageous as both a sedative/analgesic and a potential therapeutic agent for septic patients.
There are a few limitations in the present study. First, the mortality rate was not evaluated. In several studies, other anesthetics that inhibited HMGB1 release, including ketamine, lidocaine, and levobupivacaine, raised survival rates in sepsis models (23,33,37). Therefore, we could infer that remifentanil may have a similar effect on survival rate. Second, plasma levels of remifentanil were not maintained. We chose the dose of remifentanil 0.04 mg/kg in the present study because previous studies have shown that this dose of remifentanil significantly reduces inflammatory responses after CLP and lung injury after LPS-induced acute lung injury model (11,12). To reproduce a situation similar to clinical conditions, remifentanil should have been administered by continuous infusion after the inflammation occurred. We only studied the short-time effects of remifentanil on CLP-induced inflammatory cytokines, but the dose and long-term effects of remifentanil also need to be clarified. However, inhibition of inflammation in the early phase of sepsis still has implication for limiting sepsis spread. Finally, hemodynamic variables were not monitored during surgery and study drug administration. Although opioids including remifentanil have less pronounced cardiovascular effect compared to other intravenous or inhaled anesthetics, remifentanil can induce bradycardia and hypotension depending on dose. We observed the respiratory rate and occurrence of distress symptoms without invasive monitoring throughout the continuous infusion of remifentanil and recovery periods. If we had administered an arterial line, an equipment to measure the respiratory frequency and pulse oximetry, we would monitor the side effects of remifentanil precisely.
In conclusion, the present study suggested that remifentanil exhibited a protective effect against sepsis in rats. The mechanism was the suppression of HMGB1 expression by inhibition of NF-κB activity and proinflammatory cytokine release. Therefore, remifentanil is worthy of further investigation as a potential intervention strategy for multiple organ failure in sepsis. Further studies are required to elucidate the precise mechanism.
Footnotes
Funding: This research was supported by a grant from St. Vincent's hospital, Research Institute of Medical Science Foundation (SVHR-2015-6).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Seo KH, Joo JD. Data curation: Choi JW, Yoo H. Funding acquisition: Seo KH. Investigation: Seo KH, Jung HS. Writing - original draft: Seo KH. Writing - review & editing: Choi JW, Joo JD.
References
- 1.Sagy M, Al-Qaqaa Y, Kim P. Definitions and pathophysiology of sepsis. Curr Probl Pediatr Adolesc Health Care. 2013;43:260–263. doi: 10.1016/j.cppeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 2.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 4.Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, Bollaert PE. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 2007;33:1347–1353. doi: 10.1007/s00134-007-0691-2. [DOI] [PubMed] [Google Scholar]
- 5.Hou LC, Qin MZ, Zheng LN, Lu Y, Wang Q, Peng DR, Yu XP, Xin YC, Ji GL, Xiong LZ. Severity of sepsis is correlated with the elevation of serum high-mobility group box 1 in rats. Chin Med J (Engl) 2009;122:449–454. [PubMed] [Google Scholar]
- 6.Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofbauer R, Frass M, Gmeiner B, Sandor N, Schumann R, Wagner O, Kaye AD. Effects of remifentanil on neutrophil adhesion, transmigration, and intercellular adhesion molecule expression. Acta Anaesthesiol Scand. 2000;44:1232–1237. doi: 10.1034/j.1399-6576.2000.441008.x. [DOI] [PubMed] [Google Scholar]
- 10.Sacerdote P, Gaspani L, Rossoni G, Panerai AE, Bianchi M. Effect of the opioid remifentanil on cellular immune response in the rat. Int Immunopharmacol. 2001;1:713–719. doi: 10.1016/s1567-5769(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 11.Zongze Z, Jia Z, Chang C, Kai C, Yanlin W. Protective effects of remifentanil on septic mice. Mol Biol Rep. 2010;37:2803–2808. doi: 10.1007/s11033-009-9828-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Du Z, Zhou Q, Wang Y, Li J. Remifentanil attenuates lipopolysaccharide-induced acute lung injury by downregulating the NF-kappaB signaling pathway. Inflammation. 2014;37:1654–1660. doi: 10.1007/s10753-014-9893-2. [DOI] [PubMed] [Google Scholar]
- 13.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl 1):S27–38. [PubMed] [Google Scholar]
- 15.Gårdlund B, Sjölin J, Nilsson A, Roll M, Wickerts CJ, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 16.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, Thijs LG, Aarden LA. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- 18.Song XM, Wang YL, Li JG, Wang CY, Zhou Q, Zhang ZZ, Liang H. Effects of propofol on pro-inflammatory cytokines and nuclear factor kappaB during polymicrobial sepsis in rats. Mol Biol Rep. 2009;36:2345–2351. doi: 10.1007/s11033-009-9456-z. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013;62:507–514. doi: 10.1007/s00011-013-0604-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang LF, Yao YM, Sheng ZY. Novel insights for high mobility group box 1 protein-mediated cellular immune response in sepsis: a systemic review. World J Emerg Med. 2012;3:165–171. doi: 10.5847/wjem.j.issn.1920-8642.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson S, Pettilä V, Tenhunen J, Laru-Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008;34:1046–1053. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- 22.Ji MH, Zhu XL, Liu FF, Li GM, Tian M, Wu J, Fan YX, Li N, Yang JJ. Alpha 2A-adrenoreceptor blockade improves sepsis-induced acute lung injury accompanied with depressed high mobility group box-1 levels in rats. Cytokine. 2012;60:639–645. doi: 10.1016/j.cyto.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang HL, Xing YQ, Xu YX, Rong F, Lei WF, Zhang WH. The protective effect of lidocaine on septic rats via the inhibition of high mobility group box 1 expression and NF-kappaB activation. Mediators Inflamm. 2013;2013:570370. doi: 10.1155/2013/570370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang LT, Yao YM, Lu JQ, Yan XJ, Yu Y, Sheng ZY. Sodium butyrate prevents lethality of severe sepsis in rats. Shock. 2007;27:672–677. doi: 10.1097/SHK.0b013e31802e3f4c. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Stark GR. NFκB-dependent signaling pathways. Exp Hematol. 2002;30:285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- 26.Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998;83:45–56. [PubMed] [Google Scholar]
- 27.Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83:19–28. doi: 10.1016/s0165-5728(97)00217-8. [DOI] [PubMed] [Google Scholar]
- 28.Hyejin J, Mei L, Seongheon L, Cheolwon J, Seokjai K, Hongbeom B, Minsun K, Sungsu C, Sanghyun K. Remifentanil attenuates human neutrophils activation induced by lipopolysaccharide. Immunopharmacol Immunotoxicol. 2013;35:264–271. doi: 10.3109/08923973.2013.767346. [DOI] [PubMed] [Google Scholar]
- 29.Ke JJ, Zhan J, Feng XB, Wu Y, Rao Y, Wang YL. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008;36:74–78. doi: 10.1177/0310057X0803600113. [DOI] [PubMed] [Google Scholar]
- 30.von Dossow V, Luetz A, Haas A, Sawitzki B, Wernecke KD, Volk HD, Spies CD. Effects of remifentanil and fentanyl on the cell-mediated immune response in patients undergoing elective coronary artery bypass graft surgery. J Int Med Res. 2008;36:1235–1247. doi: 10.1177/147323000803600610. [DOI] [PubMed] [Google Scholar]
- 31.Winterhalter M, Brandl K, Rahe-Meyer N, Osthaus A, Hecker H, Hagl C, Adams HA, Piepenbrock S. Endocrine stress response and inflammatory activation during CABG surgery. A randomized trial comparing remifentanil infusion to intermittent fentanyl. Eur J Anaesthesiol. 2008;25:326–335. doi: 10.1017/S0265021507003043. [DOI] [PubMed] [Google Scholar]
- 32.Cho SS, Rudloff I, Berger PJ, Irwin MG, Nold MF, Cheng W, Nold-Petry CA. Remifentanil ameliorates intestinal ischemia-reperfusion injury. BMC Gastroenterol. 2013;13:69. doi: 10.1186/1471-230X-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge Y, Hu S, Zhang Y, Wang W, Xu Q, Zhou L, Mao H. Levobupivacaine inhibits lipopolysaccharide-induced high mobility group box 1 release in vitro and in vivo. J Surg Res. 2014;192:582–591. doi: 10.1016/j.jss.2014.05.087. [DOI] [PubMed] [Google Scholar]
- 34.Yu M, Shao D, Liu J, Zhu J, Zhang Z, Xu J. Effects of ketamine on levels of cytokines, NF-kappaB and TLRs in rat intestine during CLP-induced sepsis. Int Immunopharmacol. 2007;7:1076–1082. doi: 10.1016/j.intimp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Breen D, Karabinis A, Malbrain M, Morais R, Albrecht S, Jarnvig IL, Parkinson P, Kirkham AJ. Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497] Crit Care. 2005;9:R200–R210. doi: 10.1186/cc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battershill AJ, Keating GM. Remifentanil: a review of its analgesic and sedative use in the intensive care unit. Drugs. 2006;66:365–385. doi: 10.2165/00003495-200666030-00013. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Zhang L, Zhou C, Wu H. Ketamine inhibits LPS-induced HGMB1 release in vitro and in vivo. Int Immunopharmacol. 2014;23:14–26. doi: 10.1016/j.intimp.2014.08.003. [DOI] [PubMed] [Google Scholar]