Figure 3. Intravenous injection of sTRAIL LPD leads to the expression of sTRAIL in fibroblasts in situ, inhibiting stroma-vessel UMUC3/3T3 tumor growth.
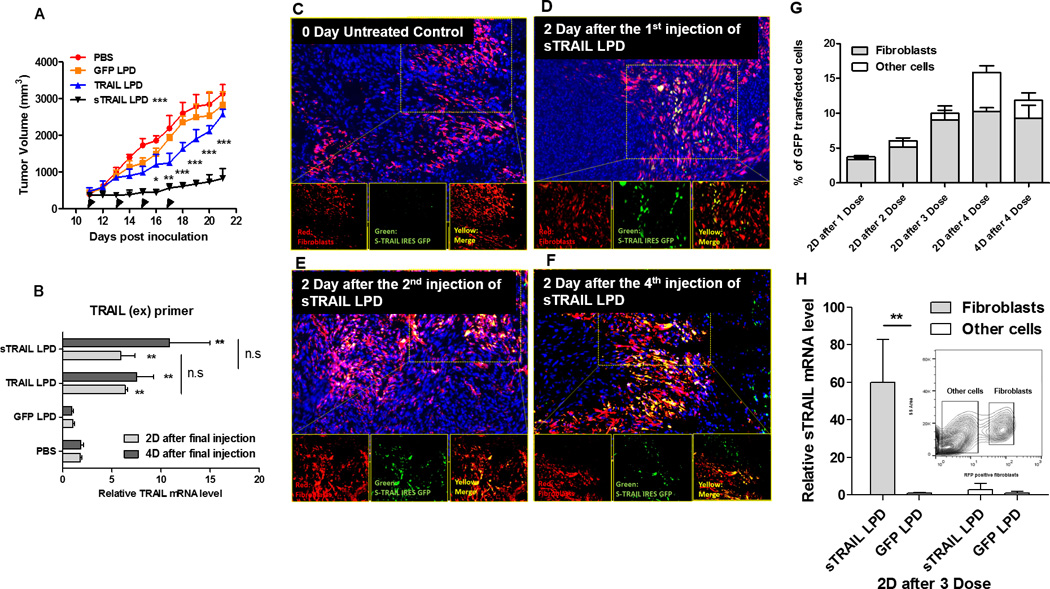
A, Tumor inhibition curve of mice bearing desmoplastic UMUC/3T3 tumors. Mice were treated with PBS, GFP LPD, TRAIL LPD or sTRAIL LPD (50 µg plasmid/mice), for 4 times (n = 6~8, * P < 0.05, ** P < 0.01, *** P < 0.001, in the legend, compared to PBS group; in the data, compared to each time point of the TRAIL LPD group). B. qPCR quantitation of relative mRNA levels of TRAIL or sTRAIL in the treated tumors. The primers simultaneously for the extracellular domain of TRAIL and sTRAIL were used for the detection (n = 6, ** P < 0.01, n.s, no significant difference, compared to PBS group). C-F, IF staining of GFP (shown green), RFP-fibroblasts (shown red) and cell nuclei (DAPI, shown blue) at indicated time points after treatments on cryo-tumor (UMUC3/3T3-RFP) tissues collected. Results showed that the majority of expression of GFP fusion protein co-localized with the RFP-labeled fibroblasts. G. Flow cytometry analysis of GFP’s association with RFP-fibroblasts at indicated time points in the dissociated cells from the collected tumor tissues (n = 4). The association of GFP in fibroblasts increased dose-dependently. H. One day after 3 doses of sTRAIL LPD, the RFP-labeled fibroblasts were sorted by flow cytometry. sTRAIL mRNA level in the RFP-labeled fibroblasts and other unlabeled cells were analyzed and compared with the GFP LPD treated group (n = 4, ** P < 0.01). The inserted chart indicates gating of the RFP fibroblasts
