Abstract
We compared two community-based HIV testing models among fisherfolk in Lake Victoria, Uganda. From May to July 2015, 1,364 fisherfolk residents of one island were offered (and 822 received) home-based testing, and 344 fisherfolk on another island were offered testing during 8 community mobilization events (outreach event-based testing). Of 207 home-based testing clients identified as HIV-positive (15% of residents), 82 were newly diagnosed, of whom 31 (38%) linked to care within 3 months. Of 41 who screened positive during event-based testing (12% of those tested), 33 were newly diagnosed, of whom 24 (75%) linked to care within 3 months. Testing costs per capita were similar for home-based ($45.09) and event-based testing ($46.99). Compared to event-based testing, home-based testing uncovered a higher number of new HIV cases but was associated with lower linkage to care. Novel community-based test-and-treat programs are needed to ensure timely linkage to care for newly diagnosed fisherfolk.
Keywords: Community-based HIV testing and counseling, Fisherfolk, Linkage to HIV care, Uganda
INTRODUCTION
Globally, only about half of people with HIV know their serostatus.1 The World Health Organization recommends conducting community-based HIV testing outside of healthcare organizations, which can increase the number of people aware of their serostatus compared to clinic-based testing alone.2–6 Healthcare facility-based (e.g., clinic) testing may not reach those who do not seek care, reside in rural areas far from facilities, lack money for transport to clinics, or cannot leave work to wait in clinics for testing.7 Community-based testing approaches are essential for key populations in sub-Saharan Africa who show high HIV prevalence and low access to care, such as Ugandan fisherfolk (fishermen and those in associated occupations such as commercial sex workers and fish traders), the focus of the present research. HIV prevalence among Ugandan Lake Victoria fisherfolk has been estimated as at least double the prevalence of the general Ugandan population (15–40%8–12 vs. 7.3%, respectively).13
In the present study, we compared two models of HIV testing for fisherfolk island communities in Lake Victoria, Uganda: outreach event-based testing and home-based testing. We examined differences between the two testing types on number of people tested, cost, and linkage to care among people who tested positive. In sub-Saharan Africa, outreach event-based testing is a standard community-based testing model, typically consisting of clinic staff conducting outreach events outside of clinic structures in communities, to educate people about HIV testing, and to provide HIV counseling and testing services.7,14,15 Event-based testing has been found to increase HIV testing uptake and to have greater geographic reach into communities than clinic-based testing, with greater potential to test individuals who do not visit healthcare centers.7 Home-based testing is another kind of community-based testing, in which testing counselors move door-to-door to test a population; it has been shown to be feasible and acceptable in higher-prevalence settings such as sub-Saharan Africa.5,16–25 Compared to event-based testing, which is more commonly conducted, home-based testing can yield higher proportions of children, first-time testers, and those who have not tested recently.4 While event-based testing has been conducted for several years on Ugandan Lake Victoria islands, home-based testing has rarely been offered to fisherfolk.9
Fisherfolk generally have low access to HIV testing due to high levels of mobility away from formal healthcare structures, stigma, and competing work priorities during testing events or clinic hours.26–29 Although community-based testing may overcome some of these barriers, the most effective method of conducting community-based testing for fisherfolk has not been determined. A primary barrier to event-based testing identified by our prior research—lack of testing due to competing work needs for individuals involved in the fish trade at the same time as testing events—suggests that different community-based models of HIV testing are needed to fully reach fisherfolk.29 For example, home-based testing may decrease barriers due to competing work needs associated with attending a public HIV-related outreach event, and therefore home-based testing (versus event-based testing) could increase the number of fisherfolk aware of their serostatus relative to event-based testing.21,30 Home-based testing may also decrease stigma concerns among fisherfolk, as home-based testing offers the option of being tested confidentially and privately in one’s own home, rather than in a public setting. Thus, we hypothesized that home-based testing (versus event-based testing) would increase the number of fisherfolk aware of their serostatus relative to event-based testing.
Few cost analyses have directly compared event-based testing to home-based testing, especially among rural populations such as fisherfolk. Studies in hard-to-reach populations in rural sub-Saharan Africa, including in Uganda, have generally found home-based testing to be less expensive than healthcare facility-based (e.g., clinic) testing. For example, a study in rural South Africa determined costs to be $29 USD per person for home-based testing versus $38 USD for clinic-based testing.22 A study in rural Uganda found that home-based HIV testing cost $5 USD per person and $54.70 USD per positive case, while facility-based HIV testing cost $6.40 USD per person and $86.50 USD per positive case.23 A study comparing costs of four HIV testing strategies in Uganda calculated costs per client (in 2007 USD) to be: $19.26 for voluntary client-initiated clinic-based testing, $11.68 for provider-initiated hospital-based testing, $13.85 for home-based testing of household members of individuals already identified as positive, and $8.29 for home-based testing.5 A systematic review found the median cost per person tested using home-based testing to be $11 USD (range = $7 to $19).31
Due to the high prevalence of HIV among fisherfolk, the Ugandan Ministry of Health recommends treatment as prevention (i.e., test-and-treat) for fisherfolk regardless of disease stage.32 Thus, the present study also provided an opportunity to explore whether linkage to care differed between the two types of community-based testing. Neither event-based nor home-based testing directly addresses linkage to care after testing, although home-based testing has been shown to increase linkage to care if it is combined with post-testing follow-up by counselors.33–35 Because prior research has not examined linkage to care after community-based testing among fisherfolk, we explored whether the two community-based testing models differed on linkage to care and did not hypothesize a direction of the effect.
In sum, we compared event-based to home-based community HIV testing on two Lake Victoria islands in Uganda. On one island, we conducted standard HIV testing mobilization events, with testing conducted in a nearby clinic during the event. On another island, we implemented home-based testing. Both models included regularly scheduled event-based ART provision on the island. We aimed to compare the two testing models on (1) number and type of people tested, (2) linkage to care, and (3) cost.
METHODS
Setting
During the present study, Mildmay Uganda, a non-governmental HIV care and treatment organization, conducted event-based HIV testing on Zzinga Island and home-based HIV testing on Kavenyanja Island, both in Lake Victoria and geographically located in the same district (Wakiso District, Uganda). Zzinga Island has about 700 households. Kavenyanja Island has about 1,100 households across Kachanga and Lwazi villages. The two islands were selected because the healthcare organization, Mildmay Uganda, had established event-based testing programs on both islands prior to the beginning of the present study.
The islands differed in their access to basic healthcare, although both received HIV testing and treatment through clinic outreach events, as described below. Both islands were in Bussi subcounty, which contains two clinics: a Health Centre III clinic on Bussi Island, and a Health Centre II clinic on Zzinga Island (where the testing events were conducted). Health Centre III clinics have a general outpatient clinic and a functioning laboratory (and the capacity to provide ART), whereas Health Centre II clinics are parish-level small outpatient clinics staffed by a nurse who provides basic preventive care; Health Centre II clinics are not accredited to offer ART. Kavenyanja Island, on which home-based testing took place, did not have a clinic. Thus, on both islands, HIV testing and treatment were only available during outreach events provided by Mildmay Uganda.
HIV Testing Protocols
General Testing Protocols
Following Ugandan policy,36 clients were considered to have screened positive if two different brands of rapid HIV tests were reactive (in serial tests). If needed, a third rapid test was used as a tie-breaker. If all three tests were weakly reactive, specimens were sent for ELISA testing. Based on Ugandan HIV testing guidelines, children aged 12–18 could assent to be tested without parental consent, and children under age 12 needed parental consent.36 Infants 18-months old or younger were tested if their mother was confirmed to be HIV-positive and provided consent. All newly diagnosed HIV-positive clients were offered a 14-day supply of cotrimoxazole and referred for ART to a clinic or the next island outreach event. To de-stigmatize HIV testing (so that healthcare workers were not associated solely with HIV), every testing client during event-based testing (on Zzinga) and household during home-based testing (on Kavenyanja) received a bed net, water purification tablets, and de-worming tablets.
Event-Based Testing
On Zzinga, rapid HIV testing was provided during eight community outreach mobilization events from May–July, 2015. Village Health Teams (VHTs) helped to mobilize and conduct outreach with fisherfolk prior to and during events (e.g., using bullhorns). VHTs are community health worker volunteers supported by the Ugandan Ministry of Health who provide health information, mobilize communities, and provide linkage to healthcare. As noted above, testing was provided during mobilization events by utilizing the space of a nearby Health Centre II. This hybrid model combined community mobilization events with clinic-based testing on specified days of the month. In contrast to clinic-based testing models, HIV testing for the present study was performed at specified times that were tied to community mobilization events, and HIV testing was not conducted during regular clinic hours.
Home-Based Testing
In Kachanga, Kavenyanja, all household residents were offered home-based testing four days per week for eight weeks (May–July 2015). Prior to the testing period, VHTs used radio announcements and home visits to inform residents of the upcoming home-based testing. Residents were eligible for testing if they had not been previously diagnosed with HIV (confirmed via ART prescription or clinic referral slips). To verify household residents and collect socio-demographic information, each household head was asked to complete a household census form. Each of three counselors took responsibility for visiting a separate section of the village, starting in residences in the outmost boundary of their section and moving toward the village center. In addition to residences, counselors visited brothels, a bar, a school, and boats inhabited by fishermen. Up to 3 return visits were made to each “household” (i.e., house, brothel, bar, school, or boat) in order to document and screen all occupants, prioritizing days and times for return visits when absent residents were more likely to be available.
Provision of ART after Testing
After both event-based testing and home-based testing, Mildmay Uganda sponsored weekly scheduled outreach events on each island to dispense ART. Specifically, ART was offered to those who had previously tested positive, regardless of disease stage. On Zzinga (event-based testing protocol), both HIV testing and treatment were provided by Mildmay Uganda at the Health Centre II clinic twice monthly, and clients who tested positive for HIV were referred to the next Mildmay Uganda outreach event at the Health Centre II on Zzinga. In Kachanga, Kavenyanja, during the home-based tested period, Mildmay Uganda conducted monthly outreach events at a church on the island, to provide ART to those who had previously tested positive on the island, and HIV testing to those who had not already been reached through home-based testing. We did not conduct home-based ART provision, because home-based ART provision would require counselors to return only to the homes of people who tested positive. This could have led to involuntary disclosure of their serostatus to others in the community (e.g., neighbors who observed that the counselors returned to some homes but not to others). Note that ART was not available on the islands outside of these scheduled events, and ART was not dispensed immediately after testing positive at the events; per the policy of the healthcare organization, clients who tested positive needed to return to the next event to initiate ART. Alternately, island residents could request to be referred for treatment to the closest Health Centre III, on Bussi Island, which had regular clinic hours for administering ART; the trip to the Bussi took about 20 minutes by boat from Zzinga and about 30 minutes by boat from Kavenyanja.
Surveys
All adults over age 18 who screened positive were invited to participate in interviews immediately following receipt of test results and three-months later. Surveys were administered on tablet computers (using Questionnaire Design Studio 3.0) in private spaces (a church or homes in Kachanga; clinic or homes on Zzinga). Participants received 10,000 Ush per survey (~$3.00 USD).
Surveys assessed socio-demographics (age, education, income, employment status, gender, marital status, occupation) and clinic-related and psychosocial correlates of linkage to HIV care, based on prior research.28,29,37–44 Perceived barriers to accessing healthcare were assessed with a checklist (alpha=.72),45,46 adapted using prior qualitative data.29 Mobility was assessed with the item, “In the past 3 months, how many times did you travel and sleep away from this community?”47 Perceived health was assessed with a validated item.48 Participants completed the internalized HIV stigma scale (alpha=.63) and items from the chronic illness anticipated stigma scale (alpha=.88) at baseline, and reported whether they had experienced 5 different types of HIV-related discrimination at follow-up.49–51 At follow-up, participants also were asked if they had disclosed to at least one person since diagnosis, if they visited a provider (i.e., linkage to care), and if they started ART.
Open-ended questions explored clients’ perceptions of testing at baseline: “What did you think of the testing? What did you think worked well? What did not work as well?” Responses were recorded, transcribed, and translated.
Administrative Data
Client Socio-demographic Characteristics
For home-based testing, household census data was reported by the head of each household, and included occupants’ gender, age, occupation, marital status, and residency status (permanent/transient). For event-based testing, counselors collected client age, gender, education level, occupation, and marital status via a standardized testing form.
Client Characteristics and Linkage to Care
We extracted from clinic records the number of clients tested and who screened positive on each island during the testing period. Because we recruited and surveyed only a portion of clients who screened positive (see below), we could not obtain data on linkage for all clients who tested positive. Thus, an HIV testing counselor telephoned all adults who tested positive for HIV to assess linkage to care three-months post-testing. Clients who could not be reached within three months were phoned again or contacted by the VHT within six-months.
Stakeholder Evaluations
We explored perceptions of home-based testing in semi-structured interviews with five key stakeholders who participated in mobilizing the community or conducting home-based testing (two VHTs, three testing counselors). Although client interviews covered both home- and event-based testing experiences, stakeholders were only asked their perceptions of home-based testing in the interviews, because it was a more novel strategy than event-based testing for island-based testing.
Institutional Review Board
The Institutional Review Boards of Boston Children’s Hospital, Makerere University School of Public Health, and the Uganda National Council for Science and Technology granted approval for the research portion of the study, which included consenting participants for the surveys and obtaining administrative testing and linkage to care data from Mildmay Uganda. The Mildmay Uganda Institutional Review Board ceded review to the Makerere University School of Public Health Institutional Review Board.
Statistical Analysis
Descriptive statistics were conducted on variables recorded in the household census form, event-based testing form, and surveys. T-tests (for continuous variables) and chi-squared tests (for categorical variables) were used to compare differences in client socio-demographic characteristics, number of HIV cases identified, and number linked to care, between the two testing protocols (using household census and event-based testing forms, and surveys), and between clients tested and those not tested through home-based testing (on household census forms). Separate bivariate and multivariate logistic regression models were used to compare socio-demographic differences between Kavenyanja island residents who were tested vs. not tested via home-based testing; to compare socio-demographic differences between home-based vs. event-based testing clients (on Kavenyanja vs. Zzinga, respectively); and using follow-up surveys, to compare differences in socio-demographic characteristics, barriers to care, mobility, perceived health, internalized stigma, discrimination, and serostatus disclosure differences between people who were diagnosed with HIV during home-based vs. event-based testing. Correlates of linkage to care across islands were examined in separate logistic regression models predicting linkage to care at follow-up with baseline socio-demographic characteristics, HIV stigma, anticipated discrimination, and anticipated disclosure (at baseline), and actual disclosure, perceived discrimination, and barriers to care (at follow-up), controlling for testing protocol.
Cost Analysis
The costs of staffing, materials, and other items for the two different testing approaches were collected from a review of project receipts and invoices, and also documented by the healthcare organization throughout the project. Fixed costs included staff salaries, per diem, and transport costs that were directly obtained from clinic budgets. (Transport costs were needed both to and from the islands, as well as between islands for overnight accommodations, because neither island had overnight lodging for the healthcare workers, and traveling back and forth to the mainland would not have been feasible in between workdays.) Other minor fixed costs included field supplies, such as plastic mats on which to conduct tests in homes, as well as life jackets and rain coats for testing counselors. These costs were calculated based on the price that the clinic paid for them. Variable costs (i.e., for each test conducted) consisted of HIV test kits and the medications and preventive items given to testing clients (i.e., de-worming tablets, bed nets, and water guard tablets). Costs are presented in terms of cost per capita and were converted from Ugandan Shillings to USD based on the exchange rate at the time they arose. The costing is therefore from the point of view of the clinic, and does not take into account costs that may have arisen on the part of the testing clients, such as transport costs to get to the testing event, or wages lost due to time spent getting tested.
Qualitative Analysis
Three team members read the majority of transcripts to identify and develop independent listings of positive and negative attitudes about home-based testing, from which a codebook was created (with a detailed description, inclusion/exclusion criteria, and typical examples of each attitudinal category). Using the data analysis program Dedoose (v6.1.18), two coders marked segments of text corresponding to each category on a randomly selected 20% of transcripts. Kappas (i.e., inter-rater reliability) ranged from 0.82–1.00 (mean=0.96) across attitudes. After attainment of inter-rater reliability, one coder applied the codebook to all passages, after which the two other team members (who had helped to develop the codebook) reviewed the coded passages and discussed and resolved any differences of opinion with the original coder.
RESULTS
Participants
Home-Based Testing
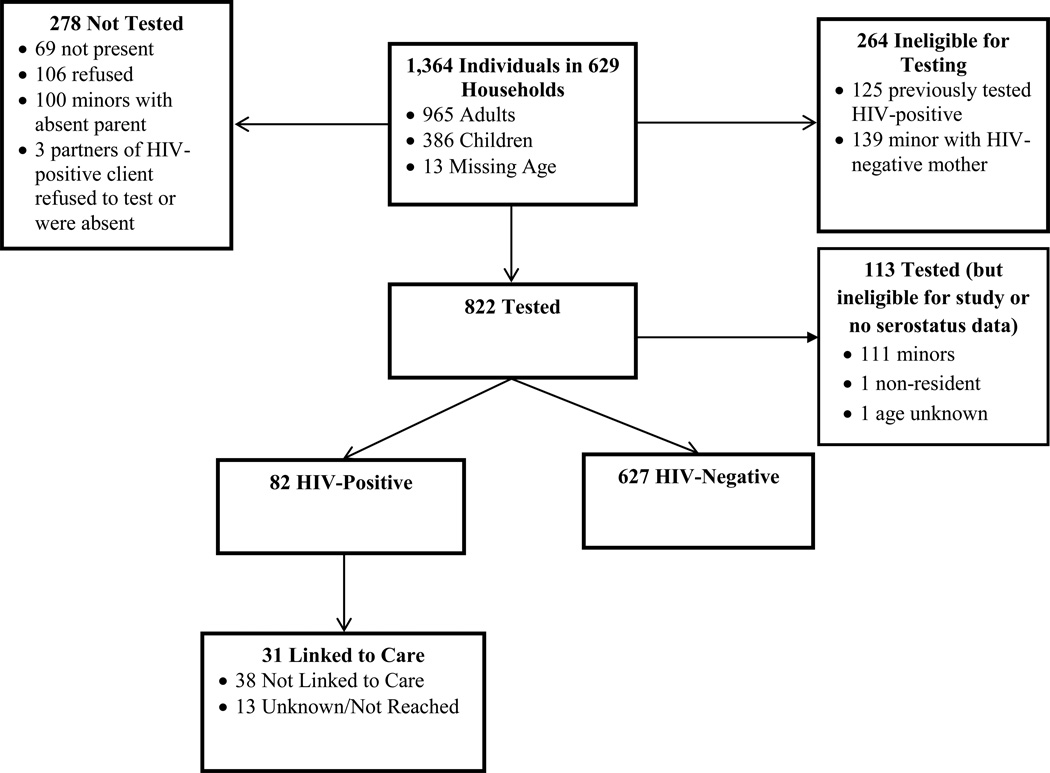
Figure 1 shows the flow chart for home-based testing, including number of people tested and not tested. Between May 13, 2015 and July 31, 2015, home-based testing was offered to 1,364 people in 629 households [556 houses, 71 non-housing structures or temporary living settings in Kachanga (1 bar, 41 boats, 26 sex workers at 8 brothels, 3 teachers at 1 school), and 2 for whom residence type was not recorded]. (Note that individuals tested at brothels and the school were considered to be from separate households.) Of those offered testing, 822 individuals (60%; 708 adults, 111 children, 3 of unknown age) were tested, 106 refused, 264 were ineligible, 69 were not present, 100 were minors (under age 12) whose parents were absent and thus could not consent, and 3 were partners of household residents who were absent or who refused testing.
Figure 1.
Flow Chart of Home-Based Testing on Kavenyanja Island, Lake Victoria (May–July, 2015)
Eighty-two clients screened positive for the first time during home-based testing, and an additional 125 confirmed a prior diagnosis and therefore were not tested during home visits. Thus, of the 947 individuals with a known serostatus in the village (i.e., 822 tested plus 125 previously diagnosed), 22% were known to be HIV-positive (15% of all residents). Based on household census data, in a multivariate model, home-based HIV testing clients were significantly more likely to be female, not married, and transient residents, compared to residents of the island who were not tested during home-based testing (e.g., not present or refused testing; see Table 1).
Table I.
Comparison of Ugandan Lake Victoria Island Residents Reached Vs. Not Reached By Home-Based HIV Testing
| Characteristic | Tested (N=822) N (%) |
Not Testeda (N=175) N (%) |
Wald χ2 statistic (df), p- valueb |
|
|---|---|---|---|---|
| Bivariate | Multivariate | |||
| Age Range (yrs.)c | χ2(1)=0.02, p=.89 |
χ2(1)=0.9, p=.35 |
||
| <18 | 111 (14) | 34 (20) | ||
| 18–30 | 404 (49) | 67 (40) | ||
| 31–40 | 207 (25) | 46 (28) | ||
| > 40 | 97 (12) | 20 (12) | ||
| Gender | χ2(1)=10.6, p=.001 |
χ2(1)=5.1, p=.02 |
||
| Male (reference) | 443 (54) | 118 (67) | ||
| Female | 379 (46) | 57 (33) | ||
| Occupationd | χ2(3)=13.9, p=.003 |
χ2(3)=5.9, p=.12 |
||
| Fisherman (reference) | 302 (37) | 74 (42) | ||
| Businessman/woman | 210 (26) | 34 (19) | ||
| Fish cleaner | 23 (3) | 4 (2) | ||
| Boat off-loader | 2 (<1) | 0 (0) | ||
| Sex worker | 12 (1) | 2 (1) | ||
| Not employed | 72 (9) | 4 (2) | ||
| Other | 200 (24) | 57 (33) | ||
| Marital Statuse | χ2(2)=13.2, p=.001 |
χ2(2)=7.1, p=.03 |
||
| Single | 191 (23) | 35 (20) | ||
| Married/Cohabitating | 470 (57) | 107 (61) | ||
| Separated/Divorced/Widowed | 85 (10) | 4 (2) | ||
| N/A | 76 (9) | 29 (17) | ||
| Residencef | χ2(1)=7.4, p=.007 |
χ2(1)=3.9, p=.04 |
||
| Permanent | 617 (75) | 147 (84) | ||
| Transient | 143 (17) | 24 (14) | ||
| Brothel | 16 (2) | 2 (1) | ||
| Floating population (e.g., boat) |
42 (5) | 0 (0) | ||
| School | 3 (<1) | 2 (1) | ||
“Not tested” includes individuals who were either absent or who refused testing, and does not include individuals who previously tested positive, and minors who were not tested because their parents were absent or their mother tested negative.
Wald χ2 statistics are estimated from logistic regression and test for association of outcome with all levels of the characteristic, with levels defined as noted below (footnotes c – f).
For comparison tests, age was tested as a continuous predictor.
For statistical tests, occupational categories were grouped as fisherman; businessman/woman, fish cleaner, boat off-loader, and sex-worker; not employed; and other.
For statistical tests, marital categories were grouped as single, divorced, separated, widowed; married/cohabitating; and N/A.
For statistical tests, residency categories were grouped as permanent and school vs. transient, brothel, and floating population.
Note: Statistical tests imputed missing data for age (missing for n=11; 1.1%), occupation (missing for n=1), and residence status (missing for n=1).
Of the 82 clients who tested positive, 60 adults completed baseline interviews, and 52 (87%) were retained at 3-month follow-up. Of the 22 HIV-positive individuals who were not interviewed, 13 were eligible (8 refused, 4 were too busy, and 1 had a language barrier), 6 were ineligible because they were children, and eligibility information was not collected for 3.
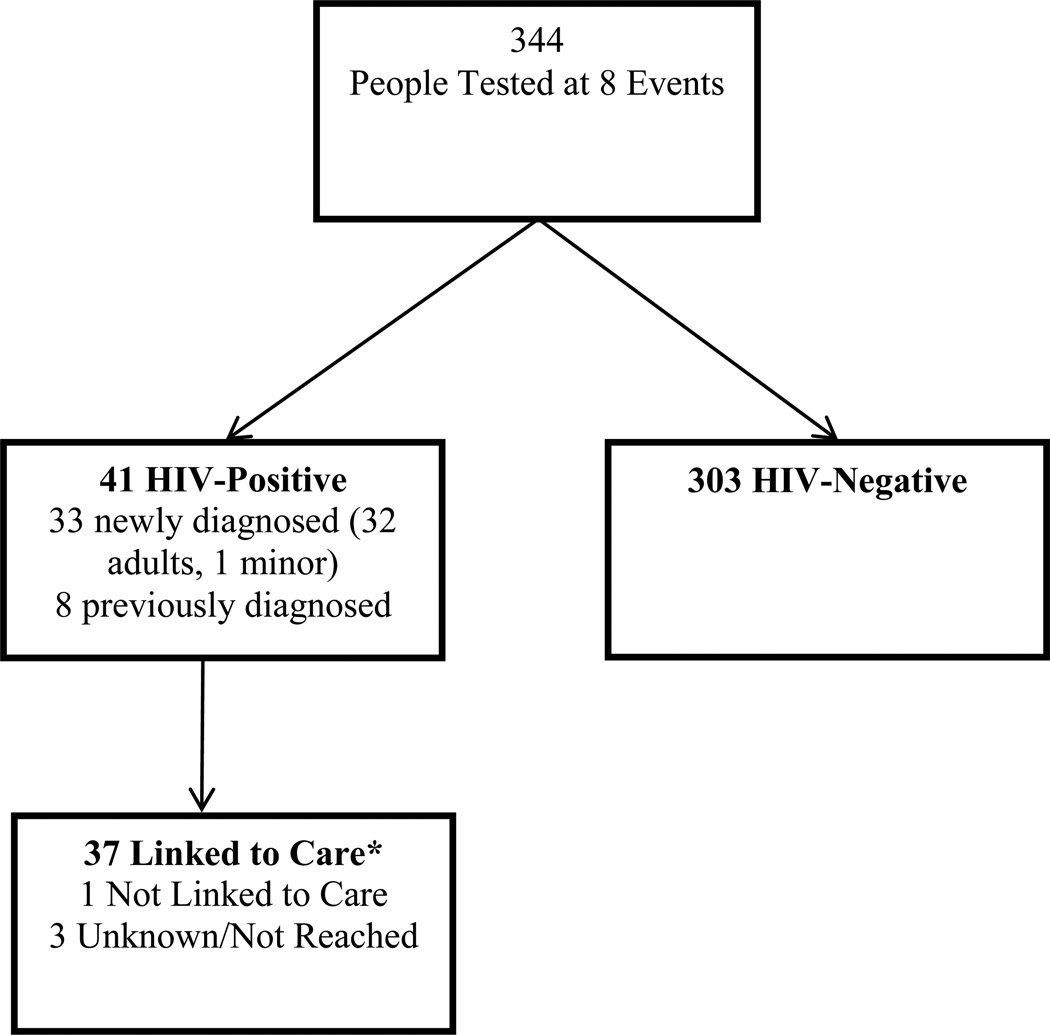
Event-Based Testing
Between May 22, 2015 and July 30, 2015, 344 clients were tested during eight events on Zzinga, of whom 41 (12%) tested positive, which included 32 newly diagnosed adults, 1 newly diagnosed child, and 8 previously diagnosed clients. Thus, 32 newly diagnosed adults were eligible for the interview, of whom 31 completed the baseline interview (1 missed the appointment), and 27 (87%) were retained (4 moved). Administrative data from testing registers was available for 248 clients who were tested; socio-demographic data were missing from the testing registers for 96 clients. Figure 2 shows the flow chart for event-based testing.
Figure 2.
Flow Chart of Outreach Event-Based Testing on Zzinga Island, Lake Victoria (May–July, 2015)
Linkage to Care
Survey Data
As noted above, all adults (aged 18 or older) who screened positive for HIV during either home-based or event-based testing were invited to participate in the survey component of the study. Among participants in the survey component, linkage to care and ART use were significantly higher after event-based than home-based testing, as indicated by separate logistic regression predicting linkage to care (or ART use) with testing protocol [89% vs. 65%, OR = 4.23 (95%CI = 1.12–16.00), p = .03; and 85% vs. 56%, OR = 4.56 (95%CI = 1.38–15.06), p = .01, respectively]. In bivariate logistic regressions, linkage to care was not significantly associated with age, sex, occupation, mobility, perceived health, barriers to care, HIV knowledge, and stigma (all p-values > .05). In addition to (and controlling for) testing protocol, only serostatus disclosure was significantly related to a higher likelihood of linkage to care three-months post-diagnosis, OR = 4.42 (95% CI = 1.41–13.92), p = 0.01.
Administrative Data
Mildmay Uganda HIV testing counselor phone calls and VHT follow-up with clients 3–6 months post-testing indicated that, of the 82 clients who tested positive after home-based testing, 31 adults (38%) had visited a provider within 3 months (and were taking ART), 38 (46%) had not visited a provider, and 13 (16%) could not be reached (Figure 1). Of the 41 adults who tested positive through event-based testing, 37 (90%) had visited a provider (of whom 36 were taking ART), 1 (2%) had not visited a provider, and 3 (7%) could not be reached (Figure 2).
Client Characteristics by Testing Protocol
Table 2 shows client socio-demographic characteristics by testing protocol, based on administrative data. In multivariate models, we examined differences between clients of home-based and event-based testing, finding that a larger proportion of home-based testing clients were children, men, fishermen, and single or married, whereas a larger proportion of event-based testing clients were over age 40, female, unemployed, and divorced, separated, or widowed.
Table II.
Comparison of Home-Based and Event-Based HIV Testing Clients on Two Ugandan Lake Victoria Islands
| Socio-Demographic Characteristics |
Event- based HIV Testing N = 248 N (%) |
Home-based HIV Testing N = 822 N (%) |
Wald χ2 statistic (df), p-value a |
|
|---|---|---|---|---|
| Bivariate | Multivariate | |||
| Age Range (yrs)b | χ2(1)=8.1, p=.005 |
χ2(1)=85.1, p<.001 |
||
| <18 | 27 (11) | 111 (14) | ||
| 18–30 | 114 (46) | 404 (49) | ||
| 31–40 | 63 (25) | 207 (25) | ||
| > 40 | 44 (18) | 97 (12) | ||
| Gender | χ2(1)=6.6, p=.01 |
χ2(1)=5.0, p=.03 |
||
| Male | 100 (45) | 443 (54) | ||
| Female | 124 (55) | 379 (46) | ||
| Occupation | χ2(3)=167.7, p<.001 |
χ2(3)=122.9, p<.001 |
||
| Fisherman | 13 (6) | 302 (37) | ||
| Fishing support services | 13 (6) | 247 (30) | ||
| Other or missing | 151 (67) | 200 (24) | ||
| Unemployed | 49 (22) | 72 (9) | ||
| Marital Statusc | χ2(2)=176.9, p<.001 |
χ2(2)=87.1, p<.001 |
||
| Single | 10 (4) | 191 (23) | ||
| Married/Cohabitating | 65 (26) | 470 (57) | ||
| Divorced/Separated/Widowed | 47 (19) | 85 (10) | ||
| N/A (minor) or missing | 126 (51) | 76 (9) | ||
Notes: Only newly diagnosed clients (who had not previously tested positive for HIV) are represented. Socio-demographic data were only available for 248 of the 344 clients tested in event-based testing, due to missing administrative data.
Statistical tests imputed missing data for age (missing for 0.2%, n = 3) and gender (2.2%, n = 24).
Wald χ2 statistics are estimated from logistic regression and test for association of outcome with all levels of the characteristic, with levels defined as noted below (footnotes b – e).
For statistical tests, age was tested as a continuous predictor.
For statistical tests, marital categories were grouped as single, divorced, separated, widowed; married/cohabitating; and N/A.
Table 3 shows a comparison by testing protocol of socio-demographic characteristics, clinic-related factors, and psychosocial variables of clients who tested positive at event-based versus home-based testing, based on survey data. Event-based and home-based testing clients who tested positive did not significantly differ on education, marital status, mobility, healthcare barriers, HIV knowledge, internalized HIV stigma, and disclosure. In multivariate models, home-based HIV-positive clients were younger, reported higher income, were more likely to be fishermen or in fishing support services, and perceived better health and greater anticipated HIV stigma at baseline. At follow-up, home-based testing clients were more likely to experience HIV-related discrimination than were event-based testing clients.
Table III.
Comparison of HIV-Positive Clients Diagnosed Via Event-Based Testing Vs. Home-Based HIV Testing on Tw Ugandan Lake Victoria Islands, Using Survey Data
| Characteristic | Event-Based HIV Testing M (SD) or N (%) |
Home-based HIV Testing M (SD) or N (%) |
Wald χ2 statistic (df)a, p-value | |
|---|---|---|---|---|
| Bivariate | Multivariate | |||
| Baseline Characteristic | N = 31 | N = 60 | ||
| Age (yrs) | 33.5 (10.1) | 31.2 (6.7) | χ2(1)=1.6, p=.20 |
χ2(1)=4.5, p=.03 |
| Education | χ2(2)=0.6, p=.74 |
χ2(2)=2.5, p=.29 |
||
| None | 3 (9.7%) | 7 (11.7%) | ||
| Primary | 22 (71.0%) | 45 (75.0%) | ||
| Secondary | 6 (19.4%) | 8 (13.3%) | ||
|
Income (Avg. Household Monthly Expenditure) |
χ2(1)=16.3, p<.001 |
χ2(1)=6.2, p=.01 |
||
| <10,000–50.999 Ush | 16 (51.6%) | 6 (10.0%) | ||
| 51,000–100.999 Ush | 15 (48.4%) | 50 (83.3%) | ||
| 101,000–>500,000 Ush |
0 (0.0%) | 4 (6.7%) | ||
| Marital Status | χ2(2)=2.2, p=.34 |
χ2(2)=0.2, p=.89 |
||
| Married/Cohabitating | 23 (74.2%) | 38 (63.3%) | ||
| Single | 4 (12.9%) | 16 (26.7%) | ||
| Separated/Divorced | 4 (12.9%) | 6 (10.0%) | ||
| Occupation | χ2(2)=9.8, p=.008 |
χ2(2)=7.3, p=.03 |
||
| Fisherman | 6 (19.4%) | 22 (36.7%) | ||
| Fishing support services |
9 (29.0%) | 32 (53.3%) | ||
| Unemployed | 12 (38.7%) | 6 (10.0%) | ||
| Other | 4 (12.9%) | 0 (0.0%) | ||
|
Mobility (# Times Traveled from Island, past 3 mos.) |
χ2(1)=3.4, p=.07 |
χ2(1)=3.2, p=.07 |
||
| 0 | 13 (44.8%) | 26 (43.3%) | ||
| 1 | 10 (34.5%) | 6 (10.0%) | ||
| 2+ | 6 (20.7%) | 28 (46.7%) | ||
|
Perceived General Health |
χ2(2)=7.1, p=.03 |
χ2(2)=9.0, p=.01 |
||
| Excellent/Very good | 2 (6.5%) | 15 (25.0%) | ||
| Good | 15 (48.4%) | 14 (23.3%) | ||
| Fair/Poor | 14 (45.2%) | 31 (51.7%) | ||
| HIV Knowledge | 5.6 (1.2) | 6.0 (1.0) | χ2(1)2.3, p = .13 |
χ2(1)=3.1, p=.08 |
| Barriers to Clinic Access | 2.2 (0.3) | 2.2 (0.5) | χ2(1)0.02, p=.88 |
χ2(1)=5.6, p=.02 |
| Internalized HIV Stigma | 1.8 (0.5) | 2.1 (0.9) | χ2(1)=3.6, p=.06 |
χ2(1)=0.7, p=.40 |
| Anticipated Stigma | 1.8 (0.7) | 2.5 (1.0) | χ2(1)=8.4, p=.004 |
χ2(1)=4.5, p=.03 |
|
Follow-Up Characteristic |
N = 27 | N = 52 | ||
|
Disclosure (to at least 1 person) |
23 (85.2%) | 37 (71.2%) | χ2(1)=1.9, p=.17 |
N/A |
| Any Discrimination | 2 (7.4%) | 19 (36.5%) | χ2(1)=6.3, p= .01 |
N/A |
Note: Statistical tests imputed missing data for mobility (n=2) and excluded the two follow-up characteristics (so that participants who were lost to follow-up were not excluded from the analysis). To achieve convergence, statistical tests combined the >100,999 Ush income category (n=4, all on Kachanga) with the 51,000–100,999 Ush category, and combined the “other” occupation category (n=4, all on Zzinga) with fishing support services. For statistical tests, mobility was treated as continuous.
Wald χ2 statistics are estimated from logistic regression and test for association of outcome with all levels of the characteristic, with levels defined as noted above.
Cost Analysis
The total costs were $62,247 for home-based testing and $25,780 for event-based testing (Table 4). The fixed costs were $33,255 for home-based and $12,890 for event-based testing, and fixed costs across both testing protocols (salaries, per diem, transport, accommodation) accounted for about 80% of the costs per test conducted. Among the fixed costs, about 25% went to salaries, 20% to transport, and 50% to per diems. The variable costs per test were higher than in other settings as in addition to the testing kits ($2.67), each client (in event-based testing) and household (in home-based testing) was given de-worming tablets ($0.20), a bed net ($3), and water guard tablets ($3.65). These added medical supplies, not typically included in HIV testing protocols, accounted for about 15% of the total per-test cost. Because the fixed costs for each testing protocol were roughly proportional to the number tested (822 for home-based, 344 for event-based), and because the rate of positive tests were similar (82 or 10% of new infections detected for home-based, 33 or 10% for event-based testing), the testing costs per capita for the two testing protocols were roughly the same: the fixed and variable costs amounted to total costs of $45.09 (home-based) and $46.99 (event-based).
Table IV.
Costs Per Capita of Home-Based vs. Event-Based Testing in Ugandan Lake Victoria Islands
| Home-Based Testing | Event-Based Testing | |
|---|---|---|
| Item | US$ | US$ |
| Salaries | 8.98 | 8.37 |
| Per-diems | 17.97 | 16.57 |
| Transport | 6.95 | 10.9 |
| Test kits | 2.67 | 2.67 |
| De-worming tablets | 0.2 | 0.2 |
| Bednets | 3 | 3 |
| Water guard tablets | 3.65 | 3.65 |
| Other (e.g., rain jackets) | 1.67 | 1.63 |
| Total per-test cost | 45.09 | 46.99 |
Acceptability of Home-Based HIV Testing
Positive perceptions of home-based HIV testing dominated open-ended responses. Stakeholders noted that clients appreciated having medical services delivered to their homes. As stated by a VHT:
“[Home-based testing] is the best form of HIV testing because the counselor can give results to someone in their home and… that wouldn't hesitate to tell you everything about him/her. However for the testing done in public one may… conceal some information. With home-based testing, that is dealt with as s/he wouldn't be afraid of anyone since s/he is inside the home and because everything is done confidentially.”
Stakeholders felt that community support was key to client acceptance of testing. For example, a counselor said:
“VHTs, they were very supportive, and they were the ones that led us to the homes, and they were the ones that introduced us. For us we’re strangers, and for them we’re a part of the community; without them, the exercise would have problems.”
As similarly reported by a VHT:
“…they knew me, their VHT, and they thought that there is no way their VHT would bring then something harmful. So it was easy for us.”
Additional advantages of home-based testing mentioned by stakeholders included the enhanced privacy and perceived further reach into populations of people unaware of their serostatus. Stakeholders had few negative experiences with home-based testing; three described instances where sero-discordant couples did not want to be counseled together.
Consistent with stakeholders, clients from event-based and home-based testing expressed satisfaction with their testing experiences. Home-based testing clients commented specifically on the convenience of being tested in their homes and indicated that they may not have been tested otherwise.
“It saved us the expenses of crossing the water and finding the right facility, and time of course, because I have a lot of work to do here and it’s hard for me to sacrifice time to go to a health facility.”—38-year old business woman
“The other thing that made me happy was that before I started getting treatment, they found me in my home and they tested me from my home. So I didn’t find any problems trekking a long journey to go and test.”— 39-year-old fisherman
DISCUSSION
The present study confirms that fisherfolk remain at high risk for HIV and demonstrates that home-based HIV testing is feasible and acceptable, and can reach a larger number of fisherfolk who are unaware of their HIV infection as compared to event-based testing within the same period of time. Home-based testing identified a greater total number of people who were previously undiagnosed than did event-based testing, yet approximately the same proportion of newly diagnosed individuals among those tested. Moreover, the costs per capita for the two testing protocols were similar.
Home-based testing was associated with lower linkage to care rates compared to event-based testing. Our relatively low rates of linkage to care for home-based testing are consistent with prior research.16 Research showing higher linkage to care rates after home-based testing than the present study included a linkage to care component, in which lay counselors followed up with clients.33,34 Thus, for any type of community-based testing outside of healthcare facilities, additional supports are needed after testing to facilitate clients’ path along the care continuum. For example, microclinic interventions, which leverage patients’ existing social networks, have shown promise in Lake Victoria island communities in improving care retention.52
Explanations for the lower linkage to care rates among home-based testing clients may be related to the population. Home-based testing may tap into a different, perhaps riskier, population than event-based testing, engaging individuals who anticipated and experienced more HIV stigma, which is related to lower engagement in care and adherence.53 Such individuals also may not have intended, or been motivated, to test at community or clinic-based events and thus may have been in a lower state of readiness for testing, disclosure, and treatment. They also may not have felt sick or been symptomatic, which can be a barrier to initiating ART.54
Another reason for lower linkage rates for home-based testing could be the differences between islands. After event-based testing, clinic staff used space in a Health Centre II to provide ART, whereas for home-based testing, clinic staff used non-clinic structures for ART provision. ART was not available in the Health Centre II outside of the HIV testing and treatment events that were evaluated for the present study. However, although testing counselors on both islands provided non-HIV medications and care in addition to ART, testing staff were associated with an HIV treatment organization, and thus clients who visited outreach events that took place in non-clinic structures might have been assumed to be HIV-positive by others in the community. The greater distance to a standing HIV clinic from the island on which we conducted home-based (vs. event-based) testing, as well as the lower frequency of ART distribution, may also help to explain why residents of this island were less likely to be linked to care. Clients may have preferred to access a clinic for treatment outside of their island of residence, due to anticipated stigma concerns about being seen getting HIV care by others on the island. Future research could determine ways to reduce stigma around ART delivery events, for example, by working with non-HIV community organizations to provide ART and other healthcare.
The costs of both home-based and event-based testing were about an order of magnitude higher than those reported in other studies in the region, due to the remoteness of the islands and their lack of infrastructure (necessitating added travel and lodging costs for clinic staff, as well as transport for medical supplies). However, the testing approaches may still be cost-effective, based on prior literature showing the benefits of voluntary HIV testing in terms of costs per infections averted or costs per disability-adjusted life-years (DALYs) saved: using the results from a free HIV testing program in Tanzania, researchers modeled the cost per infection averted to be between $92 and $170 and the costs per DALY gained to be between $4.72 and $8.72.55 In addition, based on the results from a randomized control trial of HIV testing in Kenya and Tanzania, another study estimated the cost per infection averted to be $249 in Kenya and $346 in Tanzania, and the cost per DALY saved to be $12.77 in Kenya and $17.78 in Tanzania.56 Furthermore, similar to the present study, a prevention campaign in Kenya that provided HIV testing, water filters, bed nets, and condoms in 2008 projected the cost-effectiveness of scaling up this project due to reductions in cases of diarrhea, malaria, and HIV to be less than $20 per DALY averted.57 Since our project’s costs are comparable to the upper range of costs of those in these prior studies, as a rough approximation based on Sweat et al.56 we hypothesize our cost per DALY saved to be of the order of $27.74 to $39.72—which is the DALY estimate in these studies for the ‘worst case scenario’ (i.e. high costs and low impact). Still, these costs are below the recommended threshold for the cost-effectiveness of public health interventions in less developed countries of $50 USD per DALY.58
A question raised by the relatively higher costs needed to provide life-saving services in remote settings is how to reduce costs. The first question is whether fixed costs can be reduced; given that the main cost components are salary, per diem, and transport, implementation through locally trained volunteers (rather than by clinic staff who need to travel) could be a fruitful avenue. Second, per-capita costs decrease when the number of people tested increases. Thus, determining ways to increase turnout at testing events has the potential to increase cost-effectiveness. For example, incentivizing people to come to testing events may have the potential to increase turnout, based on research that small monetary incentives can increase rates of voluntary medical male circumcision and returning for HIV test results.59,60
Several important study limitations should be noted. The islands were not randomized by testing protocol and had key differences related to population and clinic presence that could have influenced the testing population and linkage to care. We did not know the total population on the island where event-based testing was offered, and thus could not compare the percentage of the population reached by testing type. Furthermore, we could not obtain complete administrative client testing data, although the data were unlikely to be systematically missing in a way that had a high impact on results. In addition, the qualitative component comprehensively assessed perceived acceptability of the home-based testing approach only; more in-depth questions about linkage to care among both home-based and event-based testing clients would have complemented the quantitative results by exploring why linkage to care rates were lower after home-based than event-based testing. Another limitation involves the measurement of new diagnoses. Although the HIV testing counselors told household residents that only those who had not previously been diagnosed with HIV were eligible for testing, it is possible that some residents who had been previously diagnosed still volunteered to be tested (e.g., to confirm a prior diagnosis in order to obtain a clinic referral, or because they had not disclosed their serostatus to others in the household and their serostatus would be revealed if they declined to be tested). Nevertheless, regardless of individuals’ prior testing status, our home-based testing procedures helped to link all individuals who tested positive to care, by providing referrals to a nearby HIV treatment outreach event on the island.
In sum, our research suggests that home-based testing is a feasible and acceptable model for fisherfolk communities that can complement existing event-based testing models by encouraging testing among different types of clients. Future research is needed to determine how these models of community-based testing could be scaled up and paired with effective low-cost community-based linkage to care interventions, and to develop successful strategies for identifying a greater number of people living with HIV and implementing treatment as prevention for fisherfolk.
Acknowledgments
FUNDING: This study was funded by R21MH098657 and R21MH098657-02S1 from the National Institute of Mental Health (LM Bogart, Principal Investigator).
We are grateful to Rachel Akoberwa, Isabella Akol, Jak Ategeka, Eva Berinda, Fred Mabonga, David Muwanika, Patricia Pauline Nakagulire, and Umar Ssenoga for conducting interviews; Ruth Kyomuhangi, Abdul Kaweesi Mutagubya, Rashid Muyingo, Olivia Nakaweesa, Cosy Nalubega, and Justine Wateya for conducting HIV testing; and Oscar Kasozi (Mildmay Uganda Data supervisor) and Deogratius Nkugwa (Mildmay Uganda Clinical Officer); and the Village Health Teams on Kavenyanja and Zzinga islands for their contributions to community mobilization and client tracking.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS: There are no conflicts of interest to disclose. All human subjects procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.World Health Organization. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 2.World Health Organization. Progress Report 2011: Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 3.Kranzer K, Govindasamy D, van Schaik N, et al. Incentivized recruitment of a population sample to a mobile HIV testing service increases the yield of newly diagnosed cases, including those in need of antiretroviral therapy. HIV Medicine. 2012;13(2):132–137. doi: 10.1111/j.1468-1293.2011.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker LA, Jobanputra K, Rusike L, et al. Feasibility and effectiveness of two community-based HIV testing models in rural Swaziland. Trop Med Int Health. 2015;20(7):893–902. doi: 10.1111/tmi.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies N, Abang B, Wanyenze R, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23(3):395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 6.Mutale W, Michelo C, Jürgensen M, Fylkesnes K. Home-based voluntary HIV counselling and testing found highly acceptable and to reduce inequalities. BMC Public Health. 2010;10(1):1–7. doi: 10.1186/1471-2458-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matovu JK, Makumbi FE. Expanding access to voluntary HIV counselling and testing in sub-Saharan Africa: alternative approaches for improving uptake, 2001–2007. Trop Med Int Health. 2007;12(11):1315–1322. doi: 10.1111/j.1365-3156.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 8.Smolak A. A meta-analysis and systematic review of HIV risk behavior among fishermen. [2014/03/04];AIDS Care. 2014 26(3):282–291. doi: 10.1080/09540121.2013.824541. [DOI] [PubMed] [Google Scholar]
- 9.Kiwanuka N, Ssetaala A, Mpendo J, et al. High HIV-1 prevalence, risk behaviours, and willingness to participate in HIV vaccine trials in fishing communities on Lake Victoria, Uganda. J Int AIDS. Soc. 2013;16:18621. doi: 10.7448/IAS.16.1.18621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opio A, Muyonga M, Mulumba N. HIV infection in fishing communities of Lake Victoria basin of Uganda – a cross-sectional sero-behavioral survey. PLoS. ONE. 2013;8(8):e70770. doi: 10.1371/journal.pone.0070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asiki G, Mpendo J, Abaasa A, et al. HIV and syphilis prevalence and associated risk factors among fishing communities of Lake Victoria, Uganda. Sex Transm Infect. 2011 Oct;87(6):511–515. doi: 10.1136/sti.2010.046805. [DOI] [PubMed] [Google Scholar]
- 12.Kipp W, Kabwa P, Verbeck A, Fischer P, Eggert P, Buttner DW. Prevalence and risk factors of HIV-1 infection in three parishes in western Uganda. Trop Med Parasitol. 1995 Sep;46(3):141–146. [PubMed] [Google Scholar]
- 13.Uganda Ministry of Health. 2011 Uganda AIDS Indicator Survey: Preliminary Report. Kampala, Uganda: 2012. [Google Scholar]
- 14.World Health Organization. Service delivery approaches to HIV testing and counselling (HTC): a strategic policy framework. Geneva, Switzerland: 2012. [Google Scholar]
- 15.Coates TJ, Kulich M, Celentano DD, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. The Lancet Global Health. 2014;2(5):e267–e277. doi: 10.1016/S2214-109X(14)70032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tumwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care STDS. 2010 Nov;24(11):735–741. doi: 10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- 17.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-saharan Africa: a systematic review and meta-analysis. PLoS. Med. 2012;9(12):e1001351. doi: 10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyaddondo D, Wanyenze RK, Kinsman J, Hardon A. Home-based HIV counseling and testing: client experiences and perceptions in Eastern Uganda. BMC Public Health. 2012;12:966. doi: 10.1186/1471-2458-12-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14(8):849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 20.Helleringer S, Mkandawire J, Reniers G, Kalilani-Phiri L, Kohler HP. Should home-based HIV testing and counseling services be offered periodically in programs of ARV treatment as prevention? A case study in Likoma (Malawi) AIDS Behav. 2013 Jul;17(6):2100–2108. doi: 10.1007/s10461-012-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight LC, Van Rooyen H, Humphries H, Barnabas RV, Celum C. Empowering patients to link to care and treatment: qualitative findings about the role of a home-based HIV counselling, testing and linkage intervention in South Africa. AIDS Care. 2015;27(9):1162–1167. doi: 10.1080/09540121.2015.1035633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabana H, Nkonki L, Hongoro C, et al. A cost-effectiveness analysis of a home-based HIV counselling and testing intervention versus the standard (facility based) HIV testing strategy in rural South Africa. PLoS. One. 2015;10(8):e0135048. doi: 10.1371/journal.pone.0135048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulogo EM, Batwala V, Nuwaha F, Aden AS, Baine OS. Cost effectiveness of facility and home based HIV voluntary counseling and testing strategies in rural Uganda. Afr Health. Sci. 2013 Jun;13(2):423–429. doi: 10.4314/ahs.v13i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marseille E, Kahn JG, Pitter C, et al. The cost effectiveness of home-based provision of antiretroviral therapy in rural Uganda. Appl Health Econ Health Policy. 2009;7(4):229–243. doi: 10.2165/11318740-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apondi R, Bunnell R, Awor A, et al. Home-based antiretroviral care is associated with positive social outcomes in a prospective cohort in Uganda. J Acquir Immune Defic Syndr. 2007;44(1):71–76. doi: 10.1097/01.qai.0000243113.29412.dd. [DOI] [PubMed] [Google Scholar]
- 26.Mugisha E, van Rensburg GH, Potgieter E. Strategic framework for increasing accessibility and utilization of voluntary counseling and testing services in Uganda. AIDS Res Treat. 2011;2011:912650. doi: 10.1155/2011/912650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison EH, Seeley JA. HIV and AIDS among fisherfolk: a threat to ‘responsible fisheries'? Fish and Fisheries. 2004;5(3):215–234. [Google Scholar]
- 28.Gordon A. HIV/AIDS in the fisheries sector in Africa. Cairo, Egypt: World Fish Center; 2005. [Google Scholar]
- 29.Bogart LM, Naigino R, Maistrellis E, et al. Barriers to linkage to HIV care in Ugandan fisherfolk communities: a qualitative analysis. AIDS Behav. 2016;(20):2464–2476. doi: 10.1007/s10461-016-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik R, Doherty T, Jackson D, et al. Linkage to care following a home-based HIV counselling and testing intervention in rural South Africa. J Int AIDS. Soc. 2015;18:19843. doi: 10.7448/IAS.18.1.19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson C, Dalal SRB, et al. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection 2015. Geneva: World Health Organization; 2015. Systematic review of HIV testing costs in high and low income settings. [PubMed] [Google Scholar]
- 32.Uganda Ministry of Health. Addendum to the National Antiretroviral Treatment Guidelines. Uganda: 2013. [Google Scholar]
- 33.Barnabas RV, van Rooyen H, Tumwesigye E, et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. The Lancet. HIV. 2014;1(2):e68–e76. doi: 10.1016/S2352-3018(14)70024-4. 11// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64(1):e1–e8. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. [September 1, 2013];International Health. 2013 5(3):169–179. doi: 10.1093/inthealth/iht016. [DOI] [PubMed] [Google Scholar]
- 36.Uganda Ministry of Health. Uganda National Policy Guidelines for HIV Voluntary Counselling and Testing. Kampala, Uganda: Ministry of Health; 2010. [Google Scholar]
- 37.Nakigozi G, Makumbi F, Reynolds S, et al. Non-enrollment for free community HIV care: findings from a population-based study in Rakai, Uganda. AIDS Care. 2011 Jun;23(6):764–770. doi: 10.1080/09540121.2010.525614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duff P, Kipp W, Wild TC, Rubaale T, Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS. Soc. 2010;13:37. doi: 10.1186/1758-2652-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunan F. Mobility and fisherfolk livelihoods on Lake Victoria: implications for vulnerability and risk. Geoforum. 2010;41(5):776–785. 9// [Google Scholar]
- 40.Wanyenze RK, Hahn JA, Liechty CA, et al. Linkage to HIV care and survival following inpatient HIV counseling and testing. AIDS Behav. 2011;15(4):751–760. doi: 10.1007/s10461-010-9704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006 May;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kipp WE, Alibhai A, Saunders D, Konde-Lule J, Ruhunda A. Public knowledge and attitudes toward HIV/AIDS and antiretroviral therapy in Kabarole district, western Uganda. AIDS Care. 2009 Jan;21(1):118–124. doi: 10.1080/09540120802068761. [DOI] [PubMed] [Google Scholar]
- 43.Peltzer K, Friend-du Preez N, Ramlagan S, Fomundam H, Anderson J. Traditional complementary and alternative medicine and antiretroviral treatment adherence among HIV patients in Kwazulu-Natal, South Africa. Afr J Tradit Complement Altern. Med. 2010;7(2):125–137. [PMC free article] [PubMed] [Google Scholar]
- 44.Choudhry V, Ambresin AE, Nyakato VN, Agardh A. Transactional sex and HIV risks - evidence from a cross-sectional national survey among young people in Uganda. Glob Health Action. 2015;8:27249. doi: 10.3402/gha.v8.27249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bogart LM, Wagner G, Galvan FH, Banks D. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among african american men with HIV. J Acquir Immune Defic Syndr. 2010 Apr;53(5):648–655. doi: 10.1097/QAI.0b013e3181c57dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RAND Corporation. HIV Cost and Services Utilization Study. http://www.rand.org/health/projects/hcsus/Base/ [Google Scholar]
- 47.Kwena ZA, Camlin CS, Shisanya CA, Mwanzo I, Bukusi EA. Short-term mobility and the risk of HIV infection among married couples in the fishing communities along Lake Victoria, Kenya. PLoS. One. 2013;8(1):e54523. doi: 10.1371/journal.pone.0054523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009 Jan;21(1):87–93. doi: 10.1080/09540120802032627. [DOI] [PubMed] [Google Scholar]
- 50.Neuman M, Obermeyer CM. Experiences of stigma, discrimination, care and support among people living with HIV: a four country study. AIDS Behav. 2013 Jun;17(5):1796–1808. doi: 10.1007/s10461-013-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Earnshaw VA, Quinn DM, Kalichman SC, Park CL. Development and psychometric evaluation of the Chronic Illness Anticipated Stigma Scale. J Behav. Med. 2013 Jun;36(3):270–282. doi: 10.1007/s10865-012-9422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hickey MD, Salmen CR, Omollo D, et al. Implementation and operational research: pulling the network together: quasiexperimental trial of a patient-defined support network intervention for promoting engagement in HIV care and medication adherence on Mfangano Island, Kenya. J Acquir Immune Defic Syndr. 2015;69(4):e127–e134. doi: 10.1097/QAI.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS. Soc. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katz IT, Dietrich J, Tshabalala G, et al. Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS Behav. 2014;19(4):704–714. doi: 10.1007/s10461-014-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thielman NM, Chu HY, Ostermann J, et al. Cost-effectiveness of free HIV voluntary counseling and testing through a community-based AIDS service organization in Northern Tanzania. Am J Public Health. 2006;96(1):114–119. doi: 10.2105/AJPH.2004.056796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sweat M, Gregorich S, Sangiwa G, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. The Lancet. 2000;356(9224):113–121. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- 57.Kahn JG, Muraguri N, Harris B, et al. Integrated HIV testing, malaria, and diarrhea prevention campaign in Kenya: modeled health impact and cost-effectiveness. PLoS. One. 2012;7(2):e31316. doi: 10.1371/journal.pone.0031316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bank W. World Development Report 1993 : Investing in Health. New York: 1993. [Google Scholar]
- 59.Thirumurthy H, Masters SH, Rao S, et al. Effect of providing conditional economic compensation on uptake of voluntary medical male circumcision in Kenya: a randomized clinical trial. JAMA. 2014;312(7):703–711. doi: 10.1001/jama.2014.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thornton RL. The demand for, impact of, learning HIV status. The American Economic Review. 2008;98(5):1829–1863. doi: 10.1257/aer.98.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]