Abstract
Ubiquitous tyrosinase catalyses the aerobic oxidation of phenols to catechols through the binuclear copper centres. Here, inspired by the Fischer indole synthesis, we report an iridium-catalysed tyrosinase-like approach to catechols, employing an oxyacetamide-directed C–H hydroxylation on phenols. This method achieves one-step, redox-neutral synthesis of catechols with diverse substituent groups under mild conditions. Mechanistic studies confirm that the directing group (DG) oxyacetamide acts as the oxygen source. This strategy has been applied to the synthesis of different important catechols with fluorescent property and bioactivity from the corresponding phenols. Finally, our method also provides a convenient route to 18O-labelled catechols using 18O-labelled acetic acid.
Catechols are common structural motifs in bioactive molecules and synthetic building blocks. Here the authors report a method to convert phenol derivatives into catechols via an iridium-catalysed redox-neutral C–H hydroxylation, giving diversely substituted products under mild conditions.
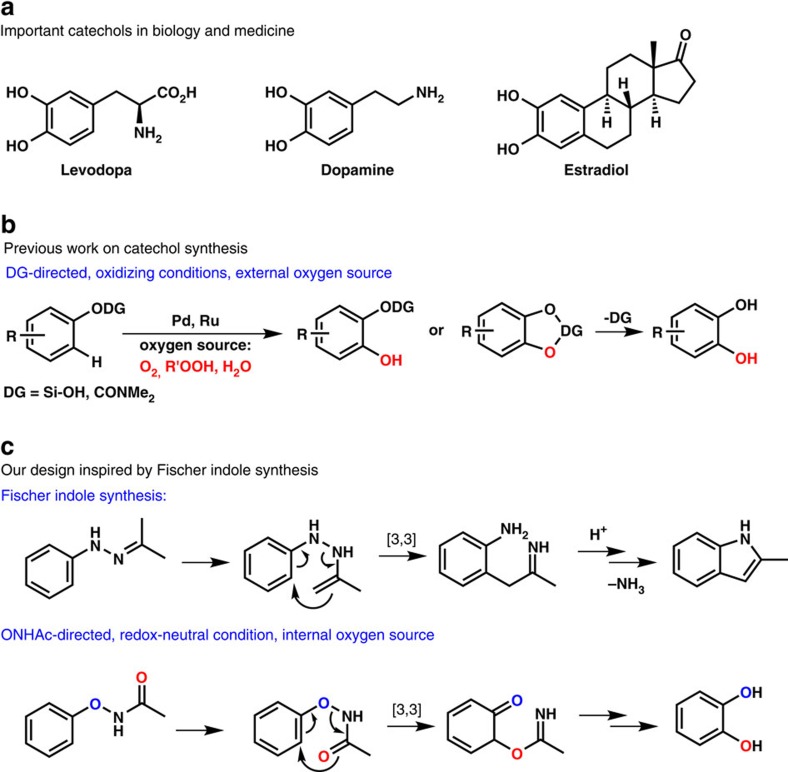
Catechols are essential bioactive molecules in human metabolism and normal physiological activities, acting as effective structural units in many bronchodilator, adrenergic, anti-parkinsonian and anti-hypertensive drugs (Fig. 1a)1. For example, L-dopa is a well-known drug used for the treatment of Parkinson's disease and is converted from L-tyrosine via catalysis by tyrosine hydroxylase in the central nervous system under mild conditions. The dopamine system plays a central role in several significant medical conditions, including attention deficit hyperactivity disorder (ADHD), schizophrenia and addictions2. On the other hand, estradiol is important in the regulation of the female oestrous and menstrual reproductive cycles3.
Figure 1. A new approach to catechol synthesis inspired by Fischer indole synthesis.
(a) Important catechols in biology and medicine. (b) Previous work on catechol synthesis. (c) Our design inspired by Fischer indole synthesis.
Synthesis of catechols usually requires lengthy steps and harsh reaction conditions, with low selectivity and conversion rates4. To develop efficient routes to synthesize catechols, we aim to construct a new C–O bond at the ortho position of phenols through directed C–H bond oxygenation. The catalytic transformation of the C–O bond from benzene to phenol is considered to be one of the greatest challenges in catalytic chemistry5. Tremendous progress has been achieved in directed C–H hydroxylation/oxygenation by metal catalysis with O2 (refs 6, 7, 8, 9), H2O (refs 10, 11, 12), peroxides13,14,15,16,17,18 or the in situ hydrolysis of newly installed acyloxy groups as oxygen sources such as OAc, OTFA and so on refs 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 (Fig. 1b). Notably, Yu and colleagues6 first developed a versatile Pd-catalysed ortho-hydroxylation of benzoic acids at 1 atm of O2 or air under non-acidic conditions. Gevorgyan and colleagues31 recently reported an ingenious silanol-directed Pd-catalysed C–H oxygenation of phenols followed by desilylation of the silacyle with tetrabutylammonium fluoride (TBAF), furnishing catechols with PhI(OAc)2 as the oxygen source. Rao and colleagues32 demonstrated the efficient ruthenium(II)- and palladium(II)-catalysed C–H hydroxylation of aryl carbamates and a subsequent deprotection to afford catechols in good yields. These pioneering works used TFA/TFAA as the oxygen source26.
We have recently reported a number of metal-catalysed C–H bond functionalizations based on a powerful directing group, -O–NHAc (oxyacetamide), which can act as an internal oxidant to yield versatile phenol derivatives33,34. A variety of substituted substrates with -O–NHAc directing group were easily prepared from phenols or aryl boronic acid compounds35,36,37,38. Inspired by the classic Fischer indole synthesis39,40,41, we hypothesized that catechols would be furnished from N-phenoxyacetamides provided that the acetamide group could function as an intramolecular oxygen source. By choosing appropriate metal catalysts, the acetamide group could function as a directing group, an internal oxidant and an oxygen source simultaneously (Fig. 1c)38,42,43,44,45. This design would avoid the need for an external oxidant, which could be detrimental to the fragile polyphenols. Notably, seminar reports from the Cheung and Buchwald46, and Zhao and colleagues47 clearly established that the acetamide group could participate in C–O bond formation.
Herein, we report an iridium-catalysed, one-step, mild and redox-neutral synthesis of catechols from acetamide-protected phenols, employing a bioinspired intramolecular oxygen transfer strategy.
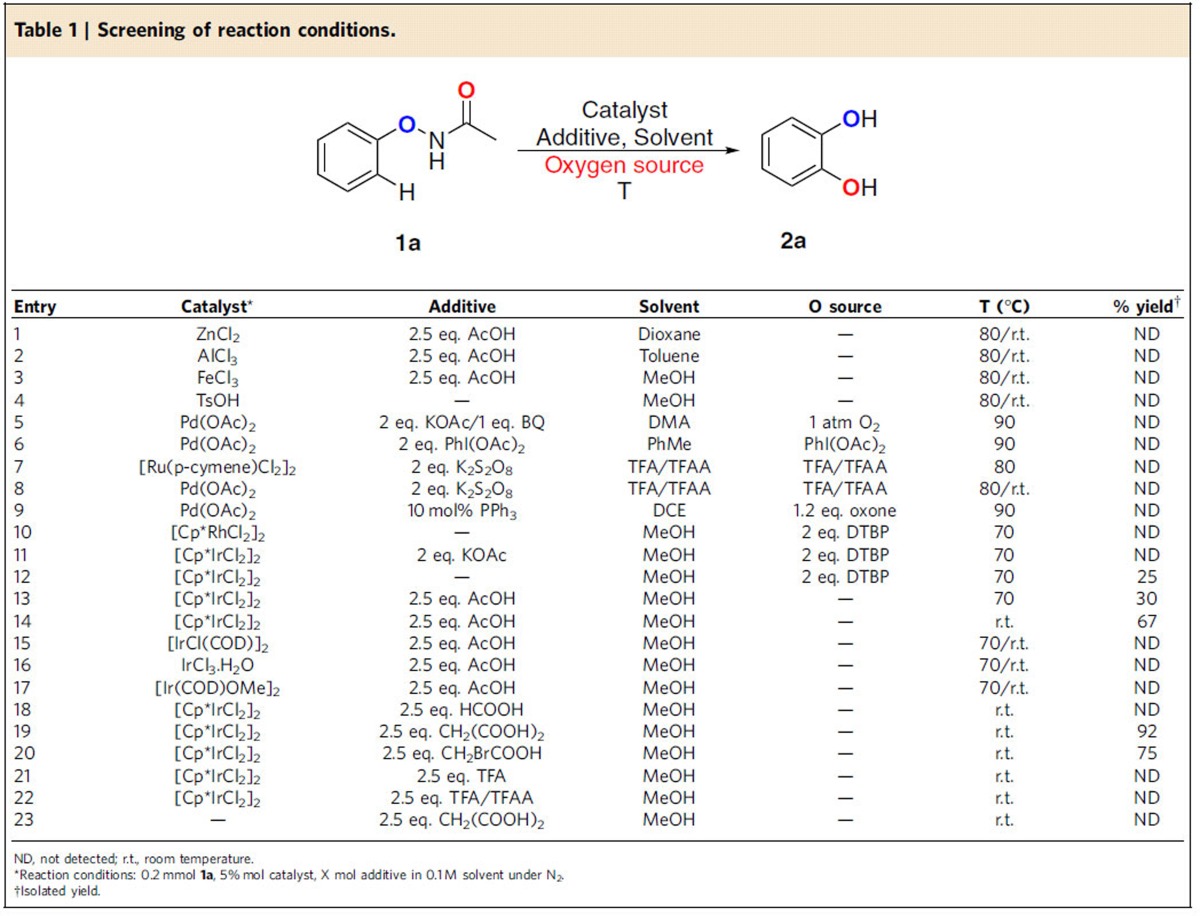
Results
Model studies
We first explored Zn-, Al-, Fe-, Cu-, Pd- and Ru-catalysed systems to N-phenoxyacetamides (1a). Unfortunately, the target product (2a) was not detected. The same result was shown in the common Rh-catalysed system for -O–NHAc (Table 1, entries 1–10 and Supplementary Table 1). Considering the design of the internal oxidation pathway, we made further attempts using other metal catalysts in the absence of an external oxidant. Gratifyingly, using 5 mol% [Cp*IrCl2]2 as catalyst and 2 equiv. of di-tert-butyl peroxide (DTBP) as a possible oxygen source and heating in MeOH at 70 °C for 12 h, the target product catechol 2a was obtained in 25% yield (Table 1, entry 12). Concurrently, a mixture of phenol byproducts from the self-decomposition of N-phenoxyacetamides was detected. According to the literature and our past experience, the reaction temperature and acid–alkali environment are important factors in the self-decomposition of N-phenoxyacetamides. It was surprising to find that target product was synthesized in the absence of DTBP when one equivalent of AcOH was added (Table 1, entry 13). Lowering the temperature greatly improved the yield. At room temperature, the reaction proceeded to give 67% yield (Table 1, entry 14). Next, various acids and alkalis were tested to improve the yield. As a whole, acids were conducive to this reaction, whereas alkalis were not. Malonic acid was preferred, with a 95% gas chromatography (GC) yield and a 92% isolated yield (Table 1, entry 19). Bromoacetic acid was slightly inferior (Table 1, entry 20), whereas HCOOH, TFA and TFA/TFAA failed to afford the product (Table 1, entries 18, 21–22). When alkali was added, no product was observed (Table 1, entry 11). In the absence of [Cp*IrCl2]2, no product was detected (Table 1, entry 23). In addition, there was no distinction under N2, O2 and air as the reaction environment. Ultimately, optimal reaction conditions to carry the ortho-hydroxylation are: 5 mol% [Cp*IrCl2]2 and 2.5 equiv. of CH2(COOH)2 in MeOH at room temperature in air with no external oxidant.
Table 1. Screening of reaction conditions.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
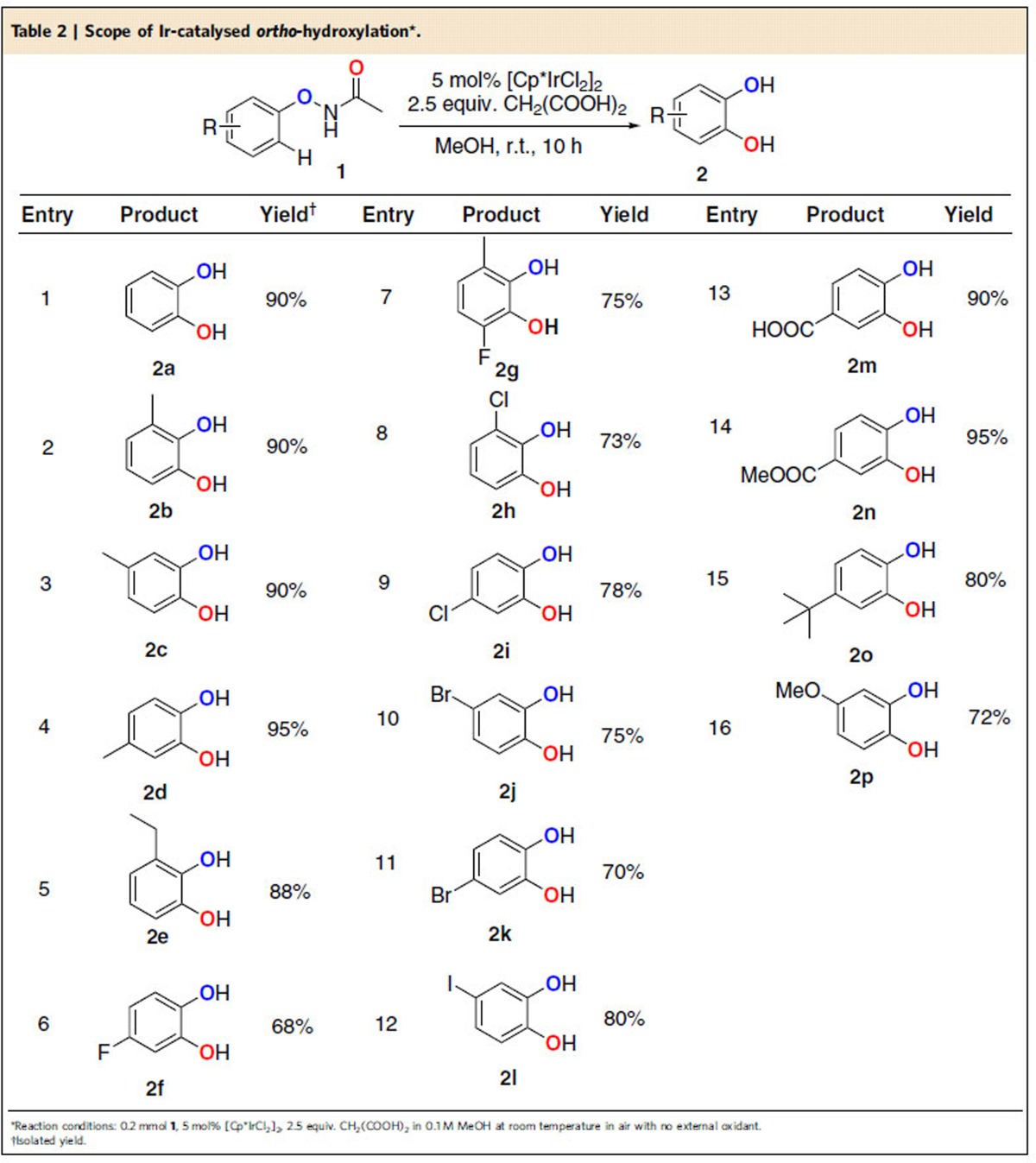
Scope of Ir-catalysed ortho-hydroxylation
The scope of the one-step neutral ortho-hydroxylation from N-phenoxyacetamides 1 to obtain catechols 2 was investigated (Table 2). Significantly, electron-neutral and electron-rich substituents such as Me, Et, tBu and OMe groups (2a–2e, 2o and 2p), all afforded excellent selectivity and good yields. The hydroxylation of substrates with electron-withdrawing substituents such as COOH, COOMe and halogen (F, Cl, Br and I with good yield) were also well tolerated (2f–2n). Ortho-, meta- and para-substituents on N-phenoxyacetamides provided similarly good yields. Remarkably, meta- and para-substituents on N-phenoxyacetamides shared the same products. This finding showed a good selectivity of hydroxylation in the ortho position, which was far away from the meta-substituents (2c and 2d, 2j and 2k). In particular, the ortho-ethyl substituent substrate showed C–H activation on the aromatic ring rather than the aliphatic chain (2e).
Table 2. Scope of Ir-catalysed ortho-hydroxylation*.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
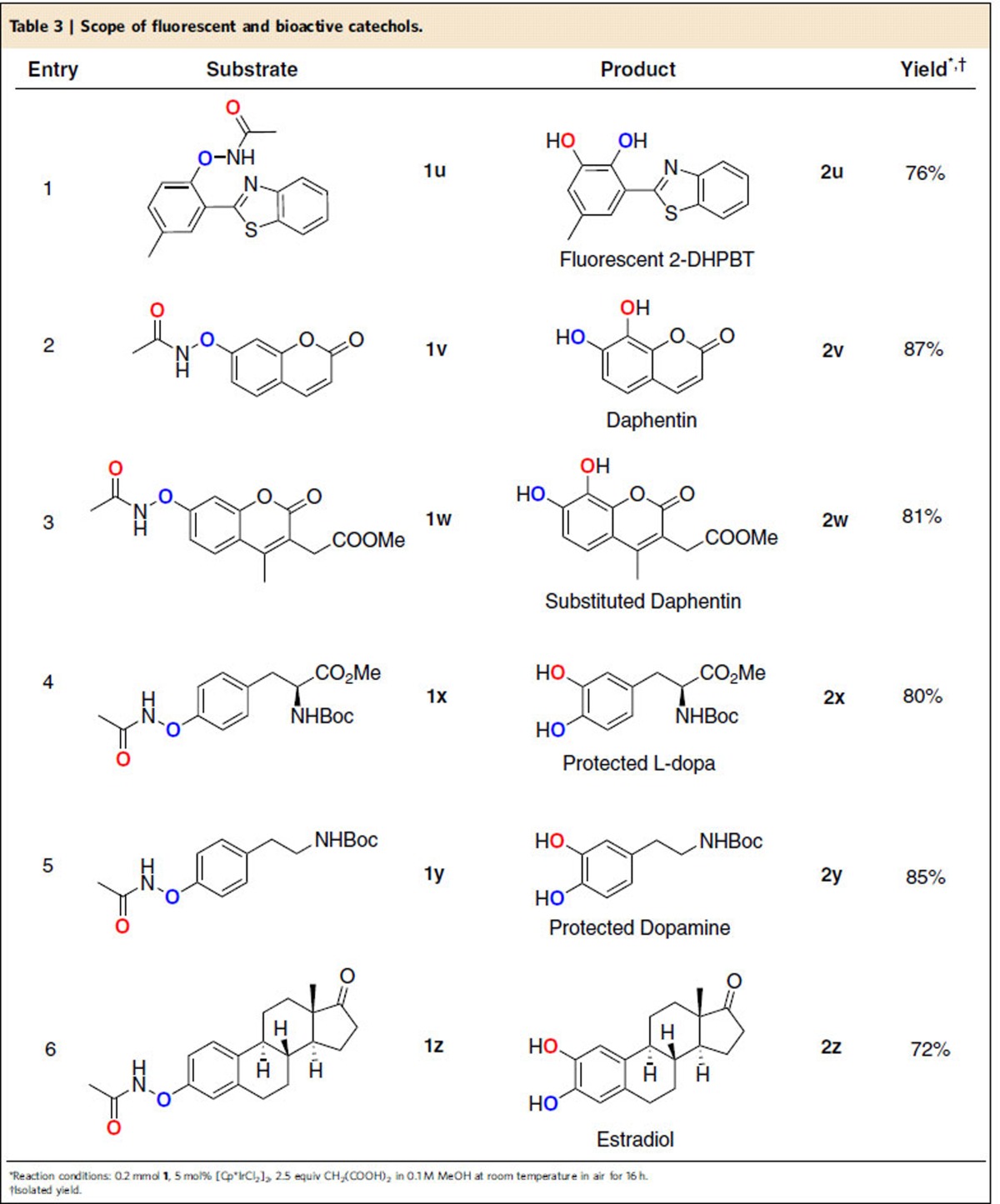
The synthesis of fluorescent and bioactive catechols
Our method provided the possibility of one-step synthesis of complicated catechols (Table 3). Recently, we reported a new method to afford different fluorescent heteroarylated phenols by N-phenoxyacetamides34. When ortho-heteroarylated N-phenoxyacetamide 1u was subject to the standard ortho-hydroxylation conditions, the desired product 2-(2,3-dihydroxyphenyl)benzothiazole derivative 2u with green fluorescence was obtained in 76% yield. Our method could be a general strategy to prepare fluorescent dihydroxyl heterocycle scaffold48,49. Next, we prepared the ONHAc-containing coumarins, the 7,8-dihydroxyl products 2v and 2w, with complete chemoselectivity in 87% and 81% yield, respectively. The product 7,8-dithydroxycoumarin 2v was commonly called Daphnetin and is an important natural product from Zushima with good anti-inflammatory and anti-oxidant activities50,51. Our reaction could provide a powerful tool in the chemical synthesis of Daphnetin derivatives. Finally, bioactive catechols natural products such as L-dopa and estradiol were tested under the standard reaction conditions, the protected L-dopa 2x, protected dopamine 2y and estradiol 2z were successfully obtained directly with high selectivity and excellent yields, highlighting the method's mild conditions,excellent functional group tolerance and selectivity.
Table 3. Scope of Ir-catalysed ortho-hydroxylation*.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
Discussion
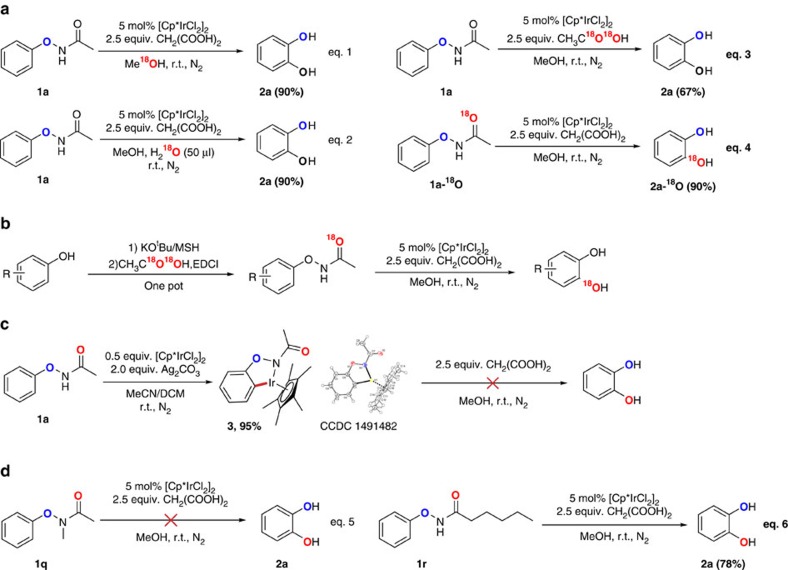
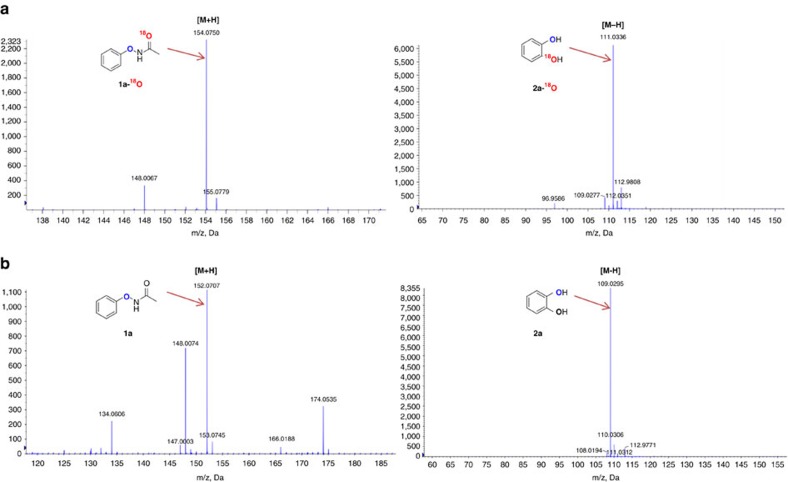
To understand the reaction mechanism, we first attempted to identify the oxygen source of the catechol products. With the standard condition, using 18O-MeOH as the solvent (Fig. 2a, eq. 1), the product detected by high resolution mass spectroscopy (HRMS) did not show the 18O-labelled catechol, which meant the oxygen of the product did not come from the solvent. After that, 50 μl 18O-H2O was added under the standard conditions (Fig. 2a, eq. 2). The results showed that the addition of water had no effect on the yield of catechol, but still no 18O-labelled product could be detected. It seemed as though that the oxygen of catechol did not come from the water that might be generated in the reaction. When 18O-labelled acetic acid (CH3C18O18OH) was added instead of malonic acid (Fig. 2a, eq. 3), the 18O-labelled product was still not detected, which showed that the acid was also not the oxygen source of the reaction. Based on the aforementioned results, we inferred that the oxygen of the product could only come from the carbonyl of the -O–NHAc group. Finally, we synthesized the substrate PhONHAc with the carbonyl labelled with 18O. Under the standard conditions, we obtained the catechol with one 18O-labelled hydroxyl (Fig. 2a, eq. 4). The 18O-labelled substrate and product were confirmed by HRMS (Fig. 3), suggesting that the reaction was an intramolecular process and that the carbonyl of the -O–NHAc group was the oxygen source. This method may be a powerful tool to access a variety of 18O-labelled catechols, which could then be used to investigate the metabolic pathways of catechol-derived bioactive compounds.
Figure 2. Mechanistic study.
(a) Isotope labelling experiments. (b) Synthesis of 18O-labelled catechols. (c) Seeking the active intermediate. (d) The importance of the N–H bond in the substrates.
Figure 3. HRMS contrast of O18-labelled and non-O18-labelled substrate and product.
(a) HRMS of O18-labelled substrate and product. (b) HRMS of non-O18-labelled substrate and product.
To probe the active intermediate in the reaction, we treated the substrate N-phenoxyacetamide (1 equiv.) and [Cp*IrCl2]2 (0.5 equiv.) with Ag2CO3 (2 equiv.) in MeCN at room temperature for 10 h, and the five-membered iridium species 3 was obtained in 95% yield. The structure of complex 3 was confirmed by nuclear magnetic resonance spectroscopy, HRMS and X-ray crystallography. However, no product was obtained when 2.5 equiv. malonic acid was added in MeOH (Fig. 2c), implying that species 3 is not an active intermediate of the reaction. Furthermore, N-methyl-substituted phenoxyacetamide 1q did not give the desired product (Fig. 2d, eq. 5) and most of the starting material was recovered. However, the reaction proceeded smoothly when the acetamide was replaced by hexanamide (Fig. 2d, eq. 6), highlighting the important role of the N–H bond.
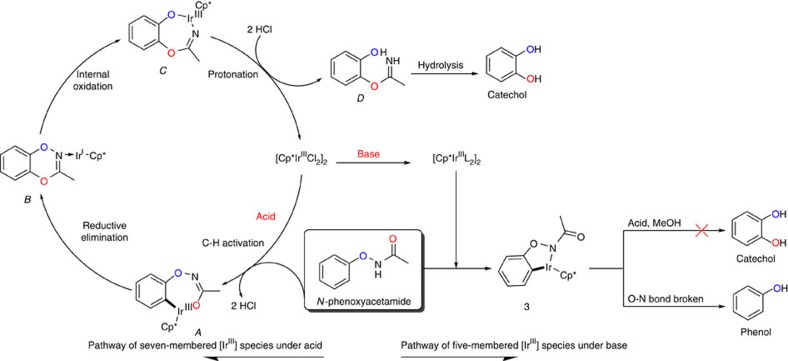
We proposed that the seven-membered [IrIII] species A might be the active intermediate, which underwent the reductive elimination process to form a C–O bond and afforded [IrI] complex B (Fig. 4). Subsequently, the O–N bond oxidatively added to the iridium complex B, generating the [IrIII] complex C. Protonation of the intermediate C regenerated the original catalyst and the imine compound D, which would be hydrolysed to catechols.
Figure 4. Proposed mechanism.
A plausible mechanism illustrating how catechols are formed in acidic condition (left) and phenols are formed in alkaline condition (right).
The acid might play several roles such as preventing the generation of inactive intermediate 3, consuming the byproduct ammonia and protonating the intermediate C. Further theoretical calculations revealed that when acid additives were introduced, the free energy of species A underwent a significant reduction from 22.4 to 2.1 kcal mol−1, highlighting the crucial role of acids in stabling species A and protonation (Supplementary Fig. 1). Mass spectrometric experiments were carried out to explore the interaction between [Cp*IrCl2]2 and different acids (HCOOH, CH2COOH and CH2(COOH)2) (Supplementary Fig. 2). Interestingly, in the Ir/HCOOH/MeOH system, HCOOH was detected losing a molecule CO2 fragment when interacted with [Cp*IrCl2]2, whereas CH2COOH or CH2(COOH)2 dissociated one molecule CH3COOH and CH2(COOH)2, respectively, by collision-induced dissociation. This result might explain why HCOOH was ineffective in promoting the reaction.
In summary, we have described the first examples of an iridium-catalysed synthesis of catechols from phenols through the formation of N-phenoxyacetamide intermediates. This bio-inspired route allows efficient, selective synthesis of catechols, which are useful building blocks for amino acids and pharmaceuticals. Our method also provides a convenient route to 18O-labelled catechols using 18O-labelled acetic acid. Further applications of the Ir/malonic acid/MeOH system and mechanistic studies of the reaction are under investigation and will be reported in due course.
Methods
Materials
For NMR spectra of compounds in this study, see Supplementary Figs 1–27. For the crystallographic data of compound 3, see Supplementary Tables 1–2 and Supplementary Fig. 28. For the representative experimental procedures and analytic data of compounds synthesized, see Supplementary Methods.
General procedure (2a)
N-phenoxyacetamide (1) (0.2 mmol), [Cp*IrCl2]2 (5 mol%) and CH2(COOH)2 (5.0 mmol) without external oxidant were weighed into a 10 ml pressure tube, to which was added anhydrous MeOH (1 ml) in a glove box. The reaction vessel was stirred at room temperature for 10 h in air. The mixture was then concentrated under vacuum and the residue was purified by column chromatography on silica gel with a gradient eluent of petroleum ether and ethyl acetate to afford the corresponding product.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC14911482. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Additional information
How to cite this article: Wu, Q. et al. A redox-neutral catechol synthesis. Nat. Commun. 8, 14227 doi: 10.1038/ncomms14227 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures, supplementary tables, supplementary discussion, supplementary methods and supplementary references.
Acknowledgments
Financial support was provided by the Shenzhen Government (JCYJ20150626110741712 and GJHZ20140417144118026), the Guangdong Government (S20120011226), the National Science Foundation of China (21622103 and 21571098) and the MOST of China (2014AA020512).
Footnotes
The authors declare no competing financial interests.
Author contributions Y.C. performed most of the reaction optimization. The experimental work was conducted by Y.C., D.Y.Y. and Q.W. Q.W. prepared most of the manuscript and supporting information. J.Z. conceived the initial ideas of this work, supervised all the experiments and coordinated with Y.L. and W.Y.S.
References
- Tyman J. H. Synthetic and Natural Phenols Vol. 52, Elsevier (1996). [Google Scholar]
- Mogwitz S., Buse J., Ehrlich S. & Roessner V. Clinical pharmacology of dopamine-modulating agents in Tourette's syndrome. Int. Rev. Neurobiol. 112, 281–349 (2013). [DOI] [PubMed] [Google Scholar]
- Ho K. Y. et al. Effects of sex and age on the 24-h profile of growth hormone secretion in man: Importance of endogenous estradiol concentrations*. J. Clin. Endocr. Metab. 64, 51–58 (1987). [DOI] [PubMed] [Google Scholar]
- Voges H. et al. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCN, New York, USA (2000).
- Haggin J. Chemists seek greater recognition for catalysis. Chem. Eng. News 71, 23–27 (1993). [Google Scholar]
- Zhang Y.-H. & Yu J.-Q. Pd(II)-catalyzed hydroxylation of arenes with 1 atm of O2 or Air. J. Am. Chem. Soc. 131, 14654–14655 (2009). [DOI] [PubMed] [Google Scholar]
- Yan Y. et al. PdCl2 and N-hydroxyphthalimide co-catalyzed C (sp2)–H hydroxylation by dioxygen activation. Angew. Chem. Int. Ed. 52, 5827–5831 (2013). [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirose D. & Taniguchi T. Direct synthesis of 1,4-diols from alkenes by iron-catalyzed aerobic hydration and C–H hydroxylation. Angew. Chem. Int. Ed. 53, 2730–2734 (2014). [DOI] [PubMed] [Google Scholar]
- Sun S. Z. et al. Cu (II)-mediated C (sp2)–H hydroxylation. J. Org. Chem. 80, 8843–8848 (2015). [DOI] [PubMed] [Google Scholar]
- Garcia-Bosch I. et al. Iron-catalyzed C–H hydroxylation and olefin cis-dihydroxylation using a single-electron oxidant and water as the oxygen-atom source. Chem. Eur. J. 18, 13269–13273 (2012). [DOI] [PubMed] [Google Scholar]
- Groger H. Hydroxy functionalization of non-activated C–H and C=C bonds: new perspectives for the synthesis of alcohols through biocatalytic processes. Angew. Chem. Int. Ed. 53, 3067–3069 (2014). [DOI] [PubMed] [Google Scholar]
- Hu J. et al. Unactivated C(sp3)-H hydroxylation through palladium catalysis with H2O as the oxygen source. Chem. Commun. 51, 14929–14932 (2015). [DOI] [PubMed] [Google Scholar]
- Chen K. & Que L. Stereospecific alkane hydroxylation by non-heme iron catalysts: mechanistic evidence for an FeVO active species. J. Am. Chem. Soc. 123, 6327–6337 (2001). [DOI] [PubMed] [Google Scholar]
- Chen K., Eschenmoser A. & Baran P. S. Strain release in C–H bond activation? Angew. Chem. Int. Ed. 48, 9705–9708 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya S., Kamijo S. & Inoue M. Direct construction of 1,3-diaxial diol derivatives by C−H hydroxylation. Org. Lett. 11, 3630–3632 (2009). [DOI] [PubMed] [Google Scholar]
- Litvinas N. D., Brodsky B. H. & Du Bois J. C-H hydroxylation using a heterocyclic catalyst and aqueous H2O2. Angew. Chem. Int. Ed. 48, 4513–4516 (2009). [DOI] [PubMed] [Google Scholar]
- Bigi M. A., Reed S. A. & White M. C. Directed metal (oxo) aliphatic C–H hydroxylations: overriding substrate bias. J. Am. Chem. Soc. 134, 9721–9726 (2012). [DOI] [PubMed] [Google Scholar]
- Hitomi Y., Arakawa K., Funabiki T. & Kodera M. An iron(III)-monoamidate complex catalyst for selective hydroxylation of alkane C–H bonds with hydrogen peroxide. Angew. Chem. Int. Ed. 51, 3448–3452 (2012). [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu V. S., Hubrich J. & Ackermann L. Ruthenium-catalyzed oxidative C(sp2)–H bond hydroxylation: site-selective C–O bond formation on benzamides. Org. Lett. 14, 4210–4213 (2012). [DOI] [PubMed] [Google Scholar]
- Yang X., Shan G. & Rao Y. Synthesis of 2-aminophenols and heterocycles by Ru-catalyzed C–H mono- and dihydroxylation. Org. Lett. 15, 2334–2337 (2013). [DOI] [PubMed] [Google Scholar]
- Choy P. Y. & Kwong F. Y. Palladium-catalyzed ortho-C-H-bond oxygenation of aromatic ketones. Org. Lett. 15, 270–273 (2013). [DOI] [PubMed] [Google Scholar]
- Li X. et al. Copper-mediated hydroxylation of arenes and heteroarenes directed by a removable bidentate auxiliary. Org. Lett. 16, 3904–3907 (2014). [DOI] [PubMed] [Google Scholar]
- Maji A., Bhaskararao B., Singha S., Sunoj R. B. & Maiti D. Directing group assisted meta-hydroxylation by C–H activation. Chem. Sci. 7, 3147–3153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. K. & Jana R. Ligand-enabled, copper-promoted regio- and chemoselective hydroxylation of arenes, aryl halides, and aryl methyl ethers. J. Org. Chem. 81, 831–841 (2016). [DOI] [PubMed] [Google Scholar]
- Wang M., Hu Y., Jiang Z., Shen H. C. & Sun X. Divergent copper-mediated dimerization and hydroxylation of benzamides involving C–H bond functionalization. Org. Biomol. Chem. 14, 4239–4246 (2016). [DOI] [PubMed] [Google Scholar]
- Shan G., Yang X., Ma L. & Rao Y. Pd-catalyzed C–H oxygenation with TFA/TFAA: expedient access to oxygen-containing heterocycles and late-stage drug modification. Angew. Chem. Int. Ed. 51, 13070–13074 (2012). [DOI] [PubMed] [Google Scholar]
- Chen K., Richter J. M. & Baran P. S. 1,3-diol synthesis via controlled, radical-mediated C−H functionalization. J. Am. Chem. Soc. 130, 7247–7249 (2008). [DOI] [PubMed] [Google Scholar]
- Mo F., Trzepkowski L. J. & Dong G. Synthesis of ortho-acylphenols through the palladium-catalyzed ketone-directed hydroxylation of arenes. Angew. Chem. Int. Ed. 51, 13075–13079 (2012). [DOI] [PubMed] [Google Scholar]
- Yang Y., Lin Y. & Rao Y. Ruthenium(II)-catalyzed synthesis of hydroxylated arenes with ester as an effective directing group. Org. Lett. 14, 2874–2877 (2012). [DOI] [PubMed] [Google Scholar]
- Yang F., Rauch K., Kettelhoit K. & Ackermann L. Aldehyde-assisted ruthenium(II)-catalyzed C–H oxygenations. Angew. Chem. Int. Ed. 53, 11285–11288 (2014). [DOI] [PubMed] [Google Scholar]
- Huang C., Ghavtadze N., Chattopadhyay B. & Gevorgyan V. Synthesis of catechols from phenols via Pd-catalyzed silanol-directed C–H oxygenation. J. Am. Chem. Soc. 133, 17630–17633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Sun Y., Chen Z. & Rao Y. A general approach towards catechol and pyrogallol through ruthenium- and palladium-catalyzed C–H hydroxylation by weak coordination. Adv. Synth. Catal. 356, 1625–1630 (2014). [Google Scholar]
- Chen Y. et al. A multitasking functional group leads to structural diversity using designer C–H activation reaction cascades. Nat. Commun. 5, 4610–4618 (2014). [DOI] [PubMed] [Google Scholar]
- Wu Q. et al. Unified synthesis of mono/bis-arylated phenols via RhIII-catalyzed dehydrogenative coupling. Chem. Sci. 8, 169–173 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Shudo K. & Okamoto T. An acid catalyzed rearrangement of O-aryl-N-benzoylhydroxylamines; synthesis of catechols from phenols. Synthesis 6, 461–463 (1980). [Google Scholar]
- Li B., Lan J., Wu D. & You J. Rhodium(III)-catalyzed ortho-heteroarylation of phenols through Internal oxidative C–H activation: rapid screening of single-molecular white-light-emitting materials. Angew. Chem. Int. Ed. 127, 14214–14218 (2015). [DOI] [PubMed] [Google Scholar]
- Petrassi H. M., Sharpless K. B. & Kelly J. W. The copper-mediated cross-coupling of phenylboronic acids and N-hydroxyphthalimide at room temperature: synthesis of aryloxyamines. Org. Lett. 3, 139–142 (2001). [DOI] [PubMed] [Google Scholar]
- Liu G., Shen Y., Zhou Z. & Lu X. Rhodium (III)-catalyzed redox-neutral coupling of N-phenoxyacetamides and alkynes with tunable selectivity. Angew. Chem. Int. Ed. 52, 6033–6037 (2013). [DOI] [PubMed] [Google Scholar]
- Robinson B. The Fischer indole synthesis. Chem. Rev. 63, 373–401 (1963). [Google Scholar]
- Wagaw S., Yang B. H. & Buchwald S. L. A palladium-catalyzed method for the preparation of indoles via the Fischer indole synthesis. J. Am. Chem. Soc. 121, 10251–10263 (1999). [Google Scholar]
- Celebi-Olcum N., Boal B. W., Huters A. D., Garg N. K. & Houk K. N. Why do some fischer indolizations fail? J. Am. Chem. Soc. 133, 5752–5755 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Liu G., Zhou Z. & Lu X. Rhodium (III)-catalyzed C–H olefination for the synthesis of ortho-alkenyl phenols using an oxidizing directing group. Org. Lett. 15, 3366–3369 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Rhodium (III)-catalyzed transannulation of cyclopropenes with N-phenoxyacetamides through C–H activation. Angew. Chem. Int. Ed. 53, 13234–13238 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou J. et al. Rh (III)-catalyzed and alcohol-involved carbenoid C–H insertion into N-phenoxyacetamides using α-diazomalonates. Chem. Commun. 51, 5868–5871 (2015). [DOI] [PubMed] [Google Scholar]
- Li B., Lan J., Wu D. & You J. Rhodium(III)-catalyzed ortho-heteroarylation of phenols through internal oxidative C–H activation: Rapid screening of single-molecular white-light-emitting materials. Angew. Chem. Int. Ed. 14, 14008–14012 (2015). [DOI] [PubMed] [Google Scholar]
- Cheung C. W. & Buchwald S. L. Room temperature copper(II)-catalyzed oxidative cyclization of enamides to 2,5-disubstituted oxazoles via vinylic C–H functionalization. J. Org. Chem. 77, 7526–7537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. et al. Synthesis of oxazoles from enamides via phenyliodine diacetate-mediated intramolecular oxidative cyclization. J. Org. Chem. 77, 10353–10361 (2012). [DOI] [PubMed] [Google Scholar]
- Charles R. G. & Freiser H. Synthesis of 2-(O-hydroxyphenyl) benzothiazole and of 2-(O- hydroxyphenyl) benzothiazoline. J. Org. Chem. 18, 422–425 (1953). [Google Scholar]
- Shindo K. et al. Synthesis of highly hydroxylated aromatics by evolved biphenyl dioxygenase and subsequent dihydrodiol dehydrogenase. Appl. Microbiol. Biot 75, 1063–1069 (2007). [DOI] [PubMed] [Google Scholar]
- Molyneux R. J. & Jurd L. The condensation of some phenols with malic acid, maleic acid and maleic anhydride. Aust. J. Chem. 27, 2697–2702 (1974). [Google Scholar]
- Fylaktakidou K. C., Hadjipavlou-Litina D. J., Litinas K. E. & Nicolaides D. N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Design 10, 3813–3833 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, supplementary tables, supplementary discussion, supplementary methods and supplementary references.
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC14911482. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.