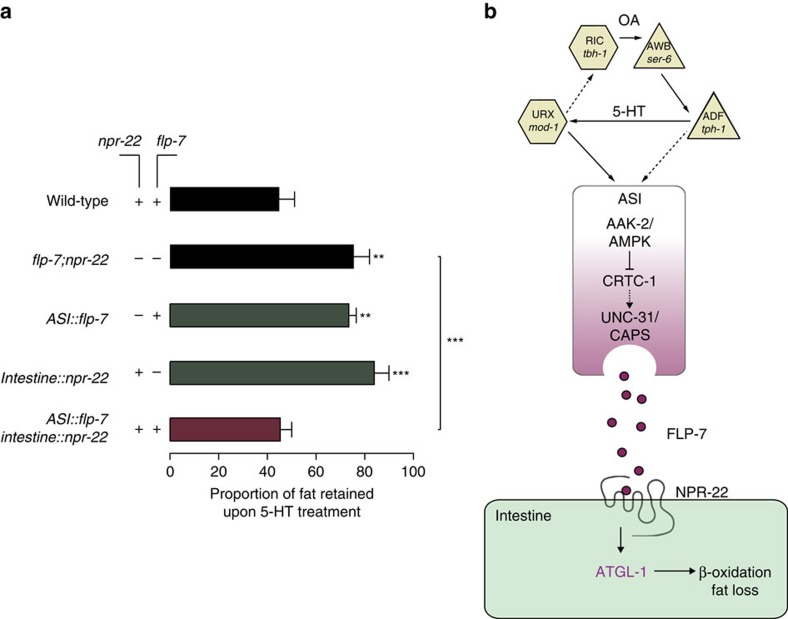
Figure 8. FLP-7 and NPR-22 function as a fat regulatory neuroendocrine ligand-receptor pair in vivo.
(a) Fat content was quantified in vehicle- and 5-HT-treated wild-type animals and in flp-7;npr-22 double mutants bearing the flp-7 and/or npr-22 transgenes under the control of the ASI daf-7 or intestinal ges-1 promoters, as indicated. Data are expressed as a proportion of fat retained upon 5-HT treatment±s.e.m. (n=11–26). **P<0.01 and ***P<0.001 by two-way ANOVA. (b) Model depicting the FLP-7/NPR-22 neuroendocrine axis that underlies the 5-HTergic control of body fat loss. In the nervous system, an integrated 5-HT and octopaminergic circuit stimulates body fat loss. In this study, we report the discovery of a tachykinin signalling system that underlies the 5-HTergic control of body fat loss in C. elegans. The FLP-7 neuroendocrine peptide is secreted from the ASI neurons in response to 5-HT and Oct-mediated signalling. The nutrient sensor AAK-2/AMPK acts in the ASI neurons via the CREB co-regulator CRTC-1 to regulate FLP-7 release in response to 5-HT-encoded signals of food availability. Upon release, FLP-7 acts in the intestine via the NPR-22/NK2R receptor to stimulate the ATGL-1 lipase, which drives fat loss. The identification of FLP-7 and NPR-22 addresses a long-standing question about the molecular basis of the central effects of 5-HT on fat loss in peripheral tissues.