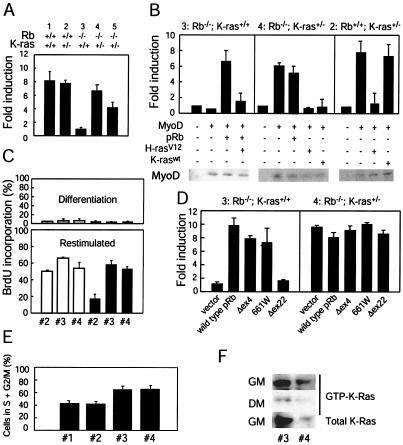
FIG. 3.
Effects of K-ras heterozygosity on MyoD transcriptional activity in Rb-deficient myoblasts. (A) MEFs of the indicated genotypes were transfected with an MCK promoter reporter construct (MCK-luc; 0.25 μg), pCSA-MyoD (1.25 μg), and pCMV-β-gal (0.25 μg). Twenty-four hours later, the cells were placed in differentiation medium for 48 h. Luciferase and β-galactosidase activities were determined, and normalized fold activations were calculated relative to the corrected luciferase activity in the absence of MyoD. Results are means ± standard errors for three independent experiments performed in triplicate. Numbers above the bar graph represent the particular MEFs used; groups 1 and 5 are derived from matched littermates, as are groups 2, 3, and 4. (B) MEFs 3, 4, and 2 were transfected as described for panel A. Plasmids encoding pRb (0.25 μg), H-rasV12 (0.5 μg), and K-raswt (0.5 μg) were included in the transfections as indicated. Fold activations were calculated relative to the corrected luciferase activity in the absence of MyoD. Results are means ± standard errors for three independent experiments performed in triplicate. Immunoblots for ectopically expressed MyoD are shown. (C) MEFs 2, 3, and 4 (designations as described for panel A) were infected with a retrovirus encoding MyoD (filled bars) or empty vector (open bars) and cultured under differentiation conditions for 72 h. Subsequently, cells were either further cultured in differentiation medium or restimulated with 20% FBS in the presence of BrdU for 24 h. Percentages of cells incorporating BrdU under differentiation conditions (upper panel) and following restimulation (lower panel) were determined. Results are means ± standard errors for two independent experiments. (D) MEFs 3 and 4 were transfected as described for panel A. A plasmid (0.25 μg) encoding pRb, pRb(661W), pRb(Δex4), or pRb(Δex22), or a vector control, was included in the transfection as indicated. Cells were treated as described for panel A, and fold activations were calculated relative to the corrected luciferase activity in the absence of MyoD. Results are means ± standard errors from three independent experiments performed in duplicate. (E) The cell cycle distribution of asynchronous cultures of MEFs 1, 2, 3, and 4 (designations as described for panel A) was determined by fluorescence-activated cell sorting. Results are means ± standard errors for percentages of cells in S and G2/M from four independent experiments. (F) MEFs 3 and 4 (designations as described for panel A) were cultured in 2% horse serum (differentiation medium [DM]) for 72 h. At this time, the level of activated, GTP-bound K-Ras was determined (middle panel). Alternatively, cells cultured in the presence of horse serum (low levels of mitogens) were restimulated with 20% fetal bovine serum (growth medium [GM]) for 6 h; at this time, the level of active K-Ras wasdetermined (top panel). Whole-cell lysates were analyzed for total K-Ras protein levels (bottom panel). Results are representative of three independent experiments.