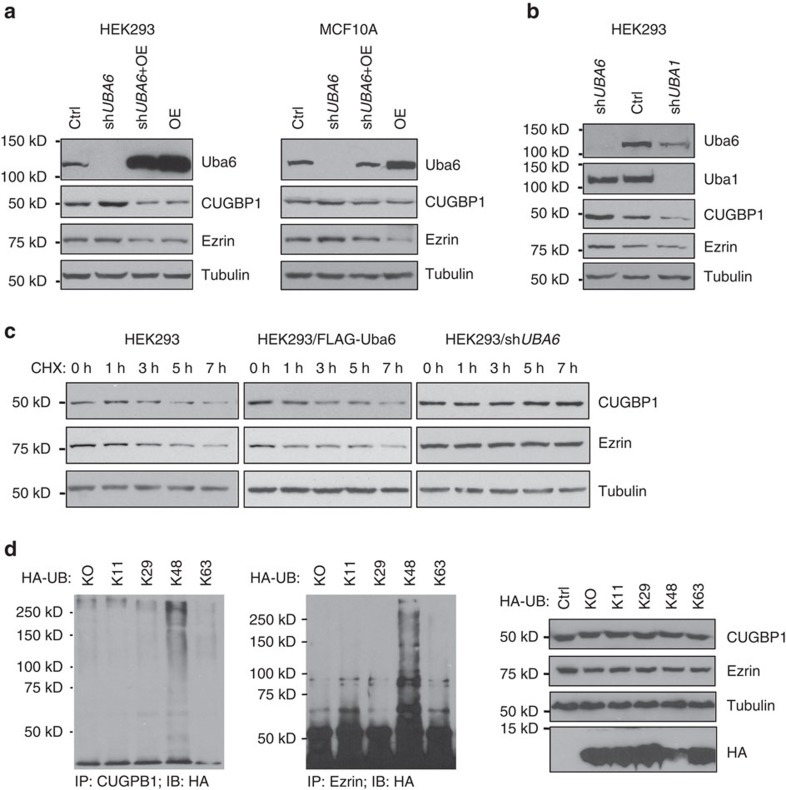
Figure 4. Uba6 negatively controls the stability of CUGBP1 and ezrin by mediating K48-linked polyubiquitination of the proteins.
(a) Effects of UBA6 silencing by shRNA (shUBA6), overexpression (OE) of Uba6, or Uba6 overexpression in the shUBA6 background (shUBA6+OE) upon the steady-state levels of the indicated proteins. HEK293 and MCF-10A cells were infected with recombinant lentiviruses to generate cell populations with Uba6 knockdown and/or overexpression, which were analysed by immunoblotting. The shRNA targets the 3′-untranslated region of the UBA6 mRNA, and does not affect exogenous cDNA expression. (b) Effects of UBA6 or UBA1 silencing on the steady-state levels of the indicated proteins determined by immunoblotting. (c) Uba6 controls the degradation of CUGBP1 and ezrin. HEK293 cells were treated with 100 μg ml−1 cycloheximide for the indicated hours, and then examined by immunoblotting for CUGBP1 or ezrin to determine its half-life. (d) Polyubiquitiated forms of CUGBP1 and ezrin consist of K48-linked ubiquitin chains. HEK293 cells were transfected with hemagglutinin (HA)-tagged ubiquitin (UB) mutants that accept chain linkage only on the K11, K29, K48 or K63 residue, followed by immunoprecipitation (IP) of the target proteins and immunoblotting (IB) for the HA tag. The right panels show cellular levels of the indicated proteins determined by direct IB.