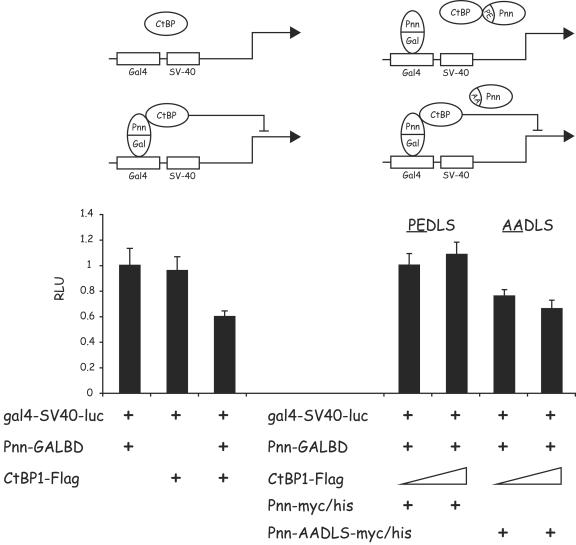
FIG. 7.
Pnn can recruit CtBP1 to a heterologous promoter, leading to a CtBP1-mediated repression of the promoter activity. (Left panel) HEK293 cells were transiently cotransfected with the luciferase reporter construct gal4-SV40-luc carrying GAL4 binding sites upstream of the SV40 constitutive promoter-enhancer and the following constructs: 100 ng of the Pnn-GALBD fusion construct alone and 1 μg of the CtBP1-Flag expression vector in the presence or absence of the Pnn-GALBD fusion construct (100 ng). gal4-SV40-luc activity was subject to CtBP1-mediated repression only in the presence of the Pnn-GALBD fusion construct, indicating that Pnn-CtBP1 interaction was responsible for this effect. (Right panel) HEK293 cells were transiently cotransfected with the gal4-SV40-luc reporter, carrying GAL4 biding sites upstream of the SV40 constitutive promoter/enhancer, 100 ng of the Pnn-GALBD fusion construct, and increasing concentrations of CtBP1-Flag (100 ng and 1 μg). In addition, transfection reactions contained 1 μg of the wild-type Pnn-myc/His or mutant form of this vector Pnn-AADLS-myc/His, carrying PE→AA substitution in the CtBP1 binding motif, as depicted in the diagram. The numerical data obtained from the luciferase readings was normalized to the value of gal4-SV40-luc reporter in the presence of Pnn-GALBD only, which represented a value of 1. The wild-type, but not mutant, form of Pnn was capable of competition with Pnn-GALBD, thereby relieving CtBP1-mediated repression of the reporter activity.