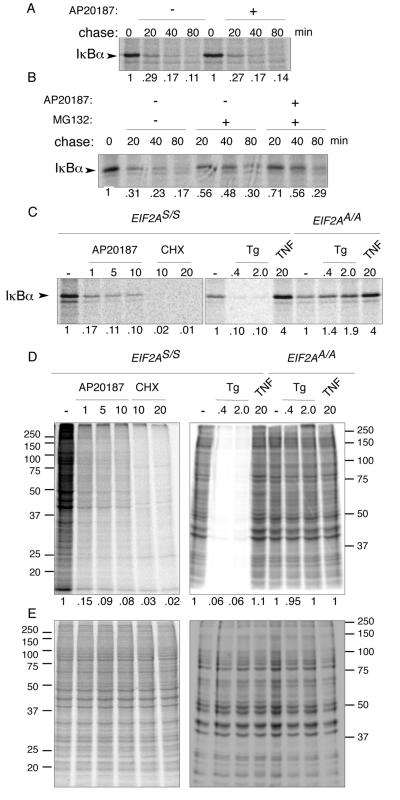
FIG. 7.
eIF2α phosphorylation inhibits synthesis of IκBα but does not destabilize the preexisting protein. (A) Autoradiogram of IκBα immunoprecipitated from wild-type (EIF2AS/S) Fv2E-PERK+ mouse fibroblasts following a brief, 10-min [35S]methionine- and cysteine-labeling pulse and cold chase of the indicated duration. The chase was conducted in the presence or absence of the activating ligand AP20187. The IκBα signal intensity is expressed as a fraction of that present at the end of the labeling pulse and is depicted beneath each lane. (B) Same assay as shown in panel A except that the proteasome inhibitor, MG132, was included during the chase where indicated. (C) Autoradiogram of the radiolabeled IκBα present at the end of the 10-min labeling pulse in wild-type (EIF2AS/S) or mutant (EIF2AA/A) Fv2E-PERK+ mouse fibroblasts treated with the indicated concentration of AP20187 ligand (in nM), cycloheximide (in μg/ml), thapsigargin (in μM), or TNF-α (in ng/ml) starting 30 min before and continuing throughout the pulse. (D) Autoradiogram ([35S]methionine) of equal fractions of the cell lysates used in panel C. The right panel is of a gel that was run longer than the left panel, accounting for differences in appearance of the two. (E) Coomassie stain of the gels shown in panel D.