Abstract
Background
After ablation of Barrett’s esophagus (BE), the esophagus heals with neosquamous epithelium (NSE). Despite normal endoscopic appearance, NSE exhibits defective barrier function with similarities to defects noted in the distal esophageal epithelium in patients with gas-troesophageal reflux disease (GERD).
Aim
To determine whether patients with NSE, unlike patients with healthy esophageal epithelium, have C-terminal fragments (CTFs) of e-cad detectable on tissue biopsy. Secondly, to determine whether patients with NSE have elevated levels of N-terminal fragments (NTFs) of e-cad in the serum.
Methods
Fifteen patients with ablated long-segment BE, who had healing with formation of NSE, were enrolled in this pilot study. Western blots for CTFs and NTFs were performed on biopsies of NSE. Venous blood was obtained to assess levels of NTFs. Endoscopic distal esophageal biopsies from patients without esophageal disease served as tissue controls. Control blood samples were obtained from healthy subjects.
Results
Blots of NSE were successful in 14/15 patients, and all 14 (100 %) had a 35-kD CTF of e-cad, while CTFs were absent in healthy control tissues. Despite CTFs in NSE, serum NTFs of e-cad in NSE were similar to controls, p > 0.05. However, unlike healthy controls, blots of NSE also showed NTFs with molecular weights of 70–90 kD.
Conclusions
Cleavage of e-cad, as evidenced by the presence of CTFs and NTFs on biopsy, contributes to defective barrier function in NSE. However, unlike findings reported in GERD patients, serum NTFs are not elevated in NSE patients. This difference may reflect poor absorption with tissue entrapment of NTFs in previously ablated areas with poorly perfused NSE.
Keywords: Barrett’s esophagus, Esophageal permeability, Western blot, ELISA, Radiofrequency ablation
Introduction
Barrett’s esophagus (BE) represents the presence of specialized columnar epithelium lining the distal esophagus [1–3]. BE is present in roughly 5–15 % of patients with gastroesophageal reflux disease (GERD), with GERD being the most recognized risk factor for its development [4–7]. Although BE is more acid-resistant than native esophageal squamous epithelium (ESE), it is a premalignant lesion, with an estimated risk of esophageal adenocarcinoma (EAC) of 0.1–0.5 %/year [8–10].
Radiofrequency ablation (RFA) is a common tool used to ablate dysplastic Barrett’s epithelium [11–15]. This technique, when combined with continuous acid suppressive therapy using proton-pump inhibitors (PPIs), results in healing of the ablated segments with a new stratified squamous epithelium called “neosquamous epithelium (NSE)” to distinguish it from native ESE [13, 16]. NSE is not just a by-product of ablation therapy, but an essential component for its success in preventing recurrence of BE. Moreover, a stable NSE also serves to prevent exposure of any submucosal islands of Barrett’s to acidic refluxate, which may in turn forestall the evolution from metaplasia to EAC [13]. The durability of NSE as replacement for BE, however, is at best uncertain as evidenced by the reported rates of recurrence of BE following ablation. For instance, recurrence of BE following successful ablation by RFA has been reported in 6–25 % of patients within 1 year [11, 15, 17–19], while recurrence of BE has been reported to occur following ablation by argon plasma coagulation (APC) in up to 66 % of patients within 15 months [20–23].
Concern about the durability of NSE has been underscored previously by our laboratory. We have observed that NSE exhibits features of defective barrier function, defects which persist in NSE for ≥2 years following ablation despite proton-pump inhibitor (PPI) therapy [16]. Specifically, NSE morphologically exhibits dilated intercellular spaces, and this in combination functionally with low transepithelial electrical resistance (RT) and high mucosal-to-serosal fluorescein flux indicates increased paracellular permeability [24–27]. Notably, these structural and functional changes are similar to those previously documented in the acid reflux-damaged ESE in patients with GERD. This suggests that NSE and reflux-damaged ESE in GERD may share other similarities with respect to their observed increases in paracellular permeability. Cleavage of the junctional protein e-cadherin is a specific defect that, in GERD, contributes to increased paracellular permeability [27]. Consequently, we hypothesized that like the reflux-damaged ESE in GERD, cleavage of e-cadherin would also be demonstrable in NSE. To test this hypothesis, we performed a pilot study to determine whether, like the ESE in GERD, cleavage of e-cadherin was evident in NSE by the presence of carboxy-terminal fragments (CTFs) of e-cad-herin in biopsies of NSE and/or there were increased levels of aminoterminal fragments (NTFs) of e-cadherin present in serum of patients with NSE.
Methods
Patient Population
We performed a prospective cohort study of adult patients, aged 18–75, who presented to the University of North Carolina (UNC) for endoscopic follow-up ≥3 months following RFA of BE. All subjects had baseline BE of ≥3 cm in length. All were taking daily PPI therapy, and all had on endoscopy complete healing of the ablated segment with NSE. Endoscopic biopsies were obtained from the area of NSE and placed in RNA-Later for determination of CTFs by Western blot. After 24 h in RNA-Later, the fixative was removed and the tissue stored at −80 °C until use. A peripheral venous blood sample was also obtained at the time of endoscopy and after allowing time to clot, it was spun down and serum decanted into vials and stored at −80 °C until use for the determination of NTFs of e-cadherin by ELISA.
Controls were adult patients, aged 18–75, who presented to UNC for diagnostic upper endoscopy and who had no history of esophageal symptoms such as heartburn, dysphagia, regurgitation, or odynophagia and no signs of esophageal abnormality on endoscopy. Esophageal biopsies were obtained from the distal 3–5 cm of esophagus and handled as for patients with NSE for Western blot analysis. Additionally, to compare serum NTFs by ELISA, serum samples were purchased from Innovative Research (Novi, MI) of healthy adult subjects that were race- and sex-matched to those of NSE patients. This study was approved by the University of North Carolina Institutional Review Board.
CTFs and NTFs of E-cadherin in Esophageal Biopsies
Western blots were performed on biopsies of NSE and from healthy ESE of controls to determine the presence of CTFs and NTFs of e-cadherin within the epithelia. The C-terminus of the linear e-cadherin protein is intracellular, while the N-terminus of e-cadherin resides in the extracellular space. Consequently, to identify CTFs remaining within the epithelial cells following cleavage, a C-terminal antibody is used. In contrast, to identify soluble N-terminal fragments of e-cadherin which following cleavage are released into the intercellular space where they potentially can be picked up by the capillary circulation, an N-terminal antibody is used (see ELISA below). The primary antibodies used for tissue were either against CTFs of human e-cadherin (Catalog# 33-4000, Zymed Laboratories, San Francisco, CA) or against NTFs of human e-cadherin (Catalog# 13-1700, Invitrogen, Camarilla, CA). Secondary antibodies were goat anti-mouse, IRDye 800 (Catalog# 610-132-121, Rockland, Gilbertsville, PA). For detection of actin (loading controls) on Western blots, we used a rabbit primary antibody (Catalog #A2066, Sigma, St. Louis) and goat anti-rabbit secondary antibody (Catalog# 611-130-122, Rockland, Gilbertsville, PA). Signals on the blot were detected using an Odyssey Infrared Imaging System (LI-COR, Inc., Lincoln, NE). Since e-cadherin has a molecular weight of 120 kD, CTFs or NTFs were recognized on Western blots as green bands with molecular weights smaller than 120 kD and quantitated with respect to actin (red bands) using densitometry by ImageJ analysis. The presence of significantly greater quantities of CTFs or NTFs on Western blots in neosquamous epithelium compared to controls was taken as evidence of cleavage of e-cadherin in the tissue.
NTFs of E-Cadherin in Serum
For quantitation of NTFs of e-cadherin in serum, an enzyme-linked immunosorbent assay (ELISA) (kit obtained from Invitrogen, Carlsbad, CA) was performed for patients with NSE and for controls. Serum for NTFs from these two groups was run as a single batch to avoid variation introduced by technique. In brief, a monoclonal antibody specific for the extracellular (N-terminus) domain of e-cadherin is coated onto microtiter plates. Samples containing an unknown amount of NTFs are incubated in the wells at 37 °C for 2 h. A second, detecting, monoclonal antibody (conjugated with peroxidase) is incubated in the wells at 37 °C for 1 h. Peroxidase substrate solution (H2O2 and tetramethylbenzidine) is added and results in a color change. The reaction is terminated by the addition of 1 M H2SO4 and absorbance of the sample measured using a microtiter plate reader at 450 nm. Each sample is measured twice and the average value used for determination of the NTFs of e-cadherin from a standard curve plotted using values obtained from standard solutions provided with the kit. Serum NTF levels from patients with NSE were compared to serum NTF levels from healthy subjects, and if elevated in NSE patients, this is taken as presumptive evidence for cleavage of e-cadherin.
Statistical Analysis
Descriptive statistics were used to summarize data, and bivariate analyses performed using Student’s t test. Student’s t test was used to compare mean serum NTFs in patients versus controls. The presence of CTFs and NTFs on Western blots for tissue samples was confirmed both qualitatively and quantitatively, the latter by densitometry performed in ImageJ and t test statistics calculated using Excel.
Results
Fifteen patients with endoscopically normal NSE and 7 endoscopic controls were enrolled in the study. The average age was 66 ± 6 years, and the subjects were predominantly male (93 % male) and all Caucasian (15/15). Slightly less than half (47 %) had GERD symptoms at the time of enrollment, and a mean of 144 ± 84 weeks had elapsed between the last RFA and study enrollment. The mean length of BE at endoscopy was 5.9 ± 2.2 cm. Two patients (13 %) were taking PPI once daily and the rest twice or more daily. Tissue controls were obtained from patients scheduled for endoscopy without esophageal symptoms or signs and biopsies obtained from grossly normal appearing distal (lower 5 cm) esophageal epithelium. Table 1 lists demographic and disease-specific characteristics of cases.
Table 1.
Demographic data of patients with neosquamous epithelium
| Age, y, mean ± SD | 66 ± 6 |
| Male, n (%) | 14 (93 %) |
| Caucasian race, n (%) | 15 (100 %) |
| GERD symptoms at baseline*, n (%) | 7 (54 %) |
| Length of BE, cm, mean ± SD | 5.9 ± 2.2 |
| Time elapsed since RFA, weeks, mean ± SD | 144 ± 84 |
Symptom history information not available for two patients
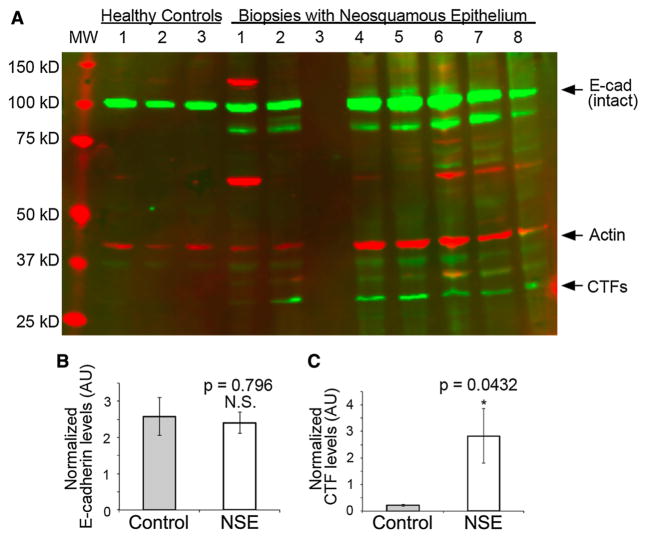
Western blots were performed on esophageal biopsies from all patients; however, the biopsies from 1 NSE patient were lost in processing so that results were available for 14 of 15 patients. Using the antibody to the C-terminus of e-cadherin, biopsies of NSE from all 14 patients and of native esophageal epithelium from all 7 controls had a prominent band at 120 kD representing intact e-cadherin. CTF bands in controls were absent or faintly detectable on Western blots, while all 14 (100 %) of the Western blots of NSE showed one or more prominent bands compatible with CTFs of e-cadherin. This is illustrated in Fig. 1a by the presence on Western blot of one or more green bands with molecular weights less than 120 kD, the most prominent being localized to a molecular weight of 35 kD. Further, as shown by densitometric analysis in Fig. 1c, quantitatively there were significantly more CTFs in neosquamous epithelium than in control tissues.
Fig. 1.
a Western blot using antibodies to the C-terminal fragments (CTFs) of e-cadherin for esophageal biopsies illustrated from 3 healthy control subjects and 7 subjects with neosquamous epithelium [specimen of neosquamous epithelium in column 3 was lost in processing]. Note that green bands for intact e-cadherin with molecular weight (MW) 120 kD are present in all 3 controls and all 7 with neosquamous epithelium. Small CTFs of e-cadherin (green bands) with MW 35 kD are shown to be absent in controls and clearly present in 6 of 7 with neosquamous epithelium. The seventh neosquamous epithelium exhibits faint, but positive, staining for CTFs of e-cadherin. Actin staining (red band) is shown at 42 kD. b E-cadherin and c CTF levels were measured by densitometry and normalized to actin. Means of the control group (healthy individuals 1–3) and experiment group (biopsies with neosquamous epithelium 1, 2, 4–8; note that biopsy 2 could not be quantified due to an anomaly in the gel lane) were plotted and compared using the t test. The error bars represent standard error. N.S. not significant
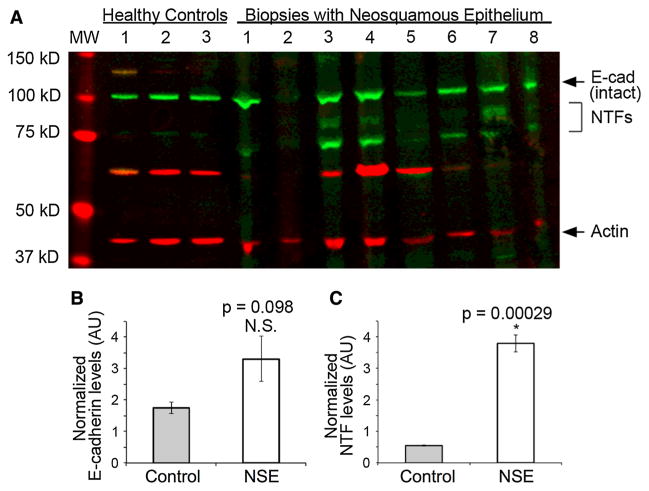
Western blots were also performed on esophageal biopsies from the 14 patients with NSE and from 3 controls using an antibody to the N-terminus of e-cadherin. Biopsies of NSE from all 14 patients and of native esophageal epithelium from the 3 controls had a prominent band at 120 kD representing intact e-cadherin. No other NTF bands were identifiable on the Western blots of controls. However, 12 of 14 (85.7 %) of the Western blots of NSE showed bands compatible with NTFs of e-cadherin. This is illustrated in Fig. 2a by the presence on Western blot of green bands with molecular weight less than 120 kD—the bands being localized between molecular weights 70 and 90 kD. Further, as shown by densitometric analysis in Fig. 2c, quantitatively there are significantly more NTFs in neosquamous epithelium than in control tissues.
Fig. 2.
a Western blot using antibodies to the N-terminal fragments (NTFs) of e-cadherin for esophageal biopsies from 3 healthy control subjects and 7 subjects with neosquamous epithelium [specimen of neosquamous epithelium in column 2 was lost in processing]. Note that green bands for intact e-cadherin with molecular weight (MW) 120 kD are present in all 3 controls and all 7 with neosquamous epithelium. NTFs of e-cadherin with MWs ranging from 70 to 90 kD (green bands) are shown to be absent in controls and clearly present in 6 of 7 with neosquamous epithelium. Actin staining (red bands) are shown at 42 kD. b E-cadherin and c NTF levels were measured by densitometry and normalized to actin. Means of the control group (healthy individuals 1–3) and experiment group (biopsies with neosquamous epithelium 3–7; note that biopsies 1, 2, 8 could not be quantified due to anomalies in the gel lanes) were plotted and compared using the t test. The error bars represent standard error. N.S. not significant
Quantification of serum NTFs by ELISA showed that the mean values for patients with NSE were similar to that of healthy controls, 67.7 ± 20 pg/ml versus 62.3 ± 19 pg/ ml, respectively, p = 0.56. There was also considerable overlap in values between groups, with NSE patients having a range of 41–104 pg/ml and controls 42–88 pg/ml (Fig. 3). Notably, there were two patients with NSE that underwent 24-h esophageal pH monitoring during the study period—with one, despite PPI therapy, being found to have elevated esophageal total acid contact time of 24.3 % and the other a normal esophageal total acid contact time of 1.5 %. Interestingly, the NSE patient with high acid contact time had high serum NTFs (70.4 pg/ml) and the NSE patient with normal acid contact time had low serum NTFs (42.7 pg/ml; Fig. 3).
Fig. 3.
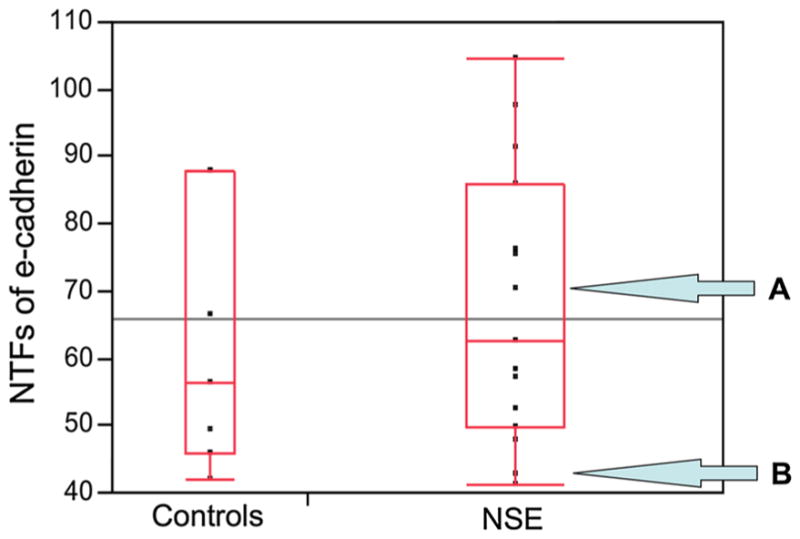
Boxplot of serum NTF levels from patients with NSE compared to serum NTF levels from healthy controls. The lines within each box represent the median values of each group. Levels obtained using enzyme-linked immunosorbent assay of N-terminal fragments (NTFs) of e-cadherin in sera from patients with neosquamous epithelium (NSE) and healthy controls, p > 0.05. Note: Patient A had a pathologically high acid contact time on esophageal pH monitoring (24.3 %) in association with the high level of NTFs in serum (70.4 pg/mL) and patient B had a normal acid contact time on pH monitoring (1.5 %) in association with a normal level of NTFs (42.7 pg/mL) in serum
Discussion
NSE, like the reflux-damaged distal esophageal epithelium in GERD patients, has defective barrier function. This is evident in both tissues morphologically by the presence of dilated intercellular spaces and functionally by a low RT and high mucosal-to-serosal fluorescein flux [16, 24–28]. These findings indicate that both NSE and the distal esophageal epithelium in GERD patients have an increase in paracellular permeability, i.e., a “leaky” route for ions and uncharged molecules between squamous epithelial cells. Recent studies in esophageal epithelium in GERD patients show that one reason for the “leaky” paracellular pathway is a defect in zonula adherens, as shown by the presence of CTFs of e-cadherin on esophageal biopsy and higher levels of NTFs of e-cadherin in serum of GERD patients [27]. In the esophageal epithelium from GERD patients, this cleavage appears to be related to the activation of “a disintegrin and metalloproteinase” (ADAM) within the squamous cell membranes, though the exact means for this activation remains unknown [27].
In the present study, we found that like the esophageal epithelium in GERD patients, NSE that arises in lower esophagus post-ablation of BE also exhibits evidence of high levels of active cleavage of e-cadherin. This was documented by the presence of increased levels of CTFs and NTFs of e-cadherin on Western blots of NSE, and the absence or very minimal presence of these fragments in Western blots of controls. The cleavage of e-cadherin in NSE, as in the esophageal epithelium in GERD patients, likely contributes to the “leakiness” of its paracellular pathway. This is because e-cadherin is the intercellular bridging protein for the zonula adherens, an essential junctional structure that completely encircles the squamous cells in stratum corneum. Moreover, the zonula adherens is located just below and supports the more luminally oriented tight junction which ultimately controls the diffusion of ions and uncharged molecules through the paracellular pathway [29–32]. Consequently, without an intact zonula adherens, the integrity of the claudin-rich tight junctions is compromised and the paracellular pathway becomes more “leaky” [31, 33].
In this study, the cleavage of e-cadherin in NSE, as in the esophageal epithelium in GERD patients, was evident by the high levels of CTFs of e-cadherin with molecular weight of 35 kD. A CTF of this size is consistent with cleavage of e-cadherin by activation of ADAM, an enzyme sheddase that cleaves at the cell membrane and releases soluble protein fractions [27, 34–36]. We have no direct evidence that activation of an ADAM in squamous cells is responsible for this component of cleavage of e-cadherin in NSE. However, given the similarities between NSE and the esophageal epithelium in GERD patients, we speculate that such activation and consequent cleavage of e-cadherin could be caused by exposure of NSE to refluxed gastric acid. However, in this study unfortunately this hypothesis could not be sufficiently tested as esophageal pH monitoring information was only available for one subject.
However, in contrast to our findings in patients with GERD, CTFs in patients with NSE were not associated with high levels of serum NTFs. The reason for this finding in our study is not clear. One possibility is that cleavage of e-cadherin occurs at lower rates in NSE than in GERD. Another explanation could be that cleaved NTFs are not able to reach the systemic circulation. RFA destroys the entire esophageal mucosa in treated areas and, in doing so, disturbs or destroys capillaries supplying blood to the area. Depending on the richness of reconstituted capillaries in NSE, soluble N-terminal fragments may have limited access to the systemic circulation. Evidence we present suggesting NTFs are detectable in tissue biopsies in NSE supports such a concept. In GERD, especially non-erosive GERD, capillary damage would not be expected to occur, and it is possible that NTFs can more easily access the systemic circulation in that milieu.
The importance of barrier function in NSE relates to its ability to serve a durable protective function against acid reflux injury, the latter being the initial cause for development of BE and presumptively for its recurrence following successful ablation. Indeed, the presence of a defective barrier would effectively lend itself to acid-induced damage to NSE by promoting acid access through the paracellular route to the basolateral membranes of squamous cells wherein lie the transport mechanisms that result in acid absorption and acidification of the cytosol of squamous cells [37]. That acid reflux plays a role in recurrence of BE following successful ablation is supported by the data of Kahaleh et al. [38], who found that 83 % of patients with recurrence of BE post-ablation by APC had pathologic acid exposure on esophageal pH monitoring compared to only 12.5 % of patients that had normal esophageal acid exposure. Moreover, the risk of acid reflux in patients with BE (and with NSE) is known to be problematic even when taking PPI therapy—with up to 50 % of BE patients continuing to experience pathologic acid reflux even while asymptomatic and on PPIs twice daily [39–41]. Taken together, these data suggest that greater attention needs to be paid to the health and integrity of NSE since its durability in part determines the post-ablation risk of BE recurrence—and by extrapolation, the need following ablation for continued endoscopic surveillance to identify recurrence of BE and repeat endoscopic treatments with RFA to avoid potential evolution from metaplasia to EAC. It is clearly better to take good care of the NSE the first time around, than to have to recurrently destroy the epithelium due to recurrent BE.
There are several limitations to this pilot study. First, the numbers of NSE patients are small and so the lack of elevated levels of NTFs in NSE as seen in GERD could reflect a type 2 error. However, given the lack of a suggestive trend in these data, this is unlikely. Second, the absence of esophageal pH probe studies limits our ability to document the role of acid reflux in the cleavage of e-cadherin. While such studies would be a welcome addition to this work, our past studies demonstrate that the addition of a pH study decreases patient willingness to participate. Third, what role the cleavage of e-cadherin plays in the recurrence of BE remains speculative. Whether this cleavage indicates the loss of a vital protective structure, or whether it is an inconsequential happenstance, or “innocent bystander,” and victim to ongoing acid damage is unclear. Nonetheless, we can conclude that the NSE that develops following RFA in patients with BE exhibits high levels of active cleavage of e-cadherin and that such cleavage likely contributes to its defective barrier function. Such defective barrier function, we propose, increases the vulnerability of NSE to acid reflux damage and as such to the risk of recurrence of BE. Given the relatively high rate of recurrence of BE after initially successful RFA, larger studies are clearly merited to establish the validity of these concepts, and to address ways to better protect the NSE.
Acknowledgments
This research was funded by T32 DK07634 (TR) and by Case Western Comprehensive Cancer Center 5U54CA163060 (Sub-Project: 5420).
Abbreviations
- BE
Barrett’s esophagus
- CTFs
C-terminal fragments
- ESE
Esophageal squamous epithelium
- NSE
Neosquamous epithelium
- GEJ
Gastroesophageal junction
- GERD
Gastroesophageal reflux disease
- NTFs
N-terminal fragments
- RFA
Radiofrequency ablation
- ELISA
Enzyme-linked immunosorbent assay
- EAC
Esophageal adenocarcinoma
- APC
Argon plasma coagulation
- RT
Transepithelial electrical resistance
Footnotes
Compliance with ethical standards
Conflict of interest Dr. Runge has no conflicts to declare. RC Orlando and Z Djukic have a patent for using the identification of fragments of e-cadherin for the diagnosis of GERD.
References
- 1.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373:850–861. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ. Barrett’s esophagus and esophageal adenocarcinoma: pathogenesis, diagnosis, and therapy. Med Clin N Am. 2002;86:1423–1445. doi: 10.1016/s0025-7125(02)00082-2. [DOI] [PubMed] [Google Scholar]
- 3.Spechler SJ. Barrett’s esophagus. In: Orlando RC, editor. Gastroesophageal Reflux Disease. New York, NY: CRC Press; 2000. pp. 219–258. [Google Scholar]
- 4.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology. 2002;123:461–467. doi: 10.1053/gast.2002.34748. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: An endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol. 2006;101:12–17. doi: 10.1111/j.1572-0241.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: Scientific review. Jama. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 9.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 10.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology. 2011;141:460–468. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyday W, Corbett F, Kuperman D, et al. Radiofrequency ablation of Barrett’s esophagus: Outcomes of 429 patients from a multi-center community practice registry. Endoscopy. 2010;42:272–278. doi: 10.1055/s-0029-1243883. [DOI] [PubMed] [Google Scholar]
- 13.Pouw RE, Gondrie JJ, Rygiel AM, et al. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am J Gastroenterol. 2009;104:1366–1373. doi: 10.1038/ajg.2009.88. [DOI] [PubMed] [Google Scholar]
- 14.Herrero LA, Pouw RE, Van Vilsteren FG, et al. What are the outcomes of endoscopic radiofrequency ablation for very long segments of Barrett esophagus containing neoplasia? Gastrointest Endosc. 2009;69:AB116. [Google Scholar]
- 15.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 16.Jovov B, Shaheen NJ, Orlando GS, et al. Defective barrier function in neosquamous epithelium. Am J Gastroenterol. 2013;108:386–391. doi: 10.1038/ajg.2012.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:1840–1847. doi: 10.1016/j.cgh.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of intestinal metaplasia after successful eradication of Barrett’s esophagus with radiofrequency ablation. Dig Dis Sci. 2011;56:1996–2000. doi: 10.1007/s10620-011-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: Results from a US Multicenter Consortium. Gastroenterology. 2013;145:79–86. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 21.Basu K, Pick B, Bale R, et al. Efficacy and one year follow up of argon plasma coagulation therapy for ablation of Barrett’s oesophagus: Factors determining persistence and recurrence of Barrett’s epithelium. Gut. 2002;51:776–780. doi: 10.1136/gut.51.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michopoulos S, Tsibouris P, Bouzakis H, et al. Complete regression of Barrett’s esophagus with heat probe thermocoagulation: Mid-term results. Gastrointest Endosc. 1999;50:165–172. doi: 10.1016/s0016-5107(99)70219-1. [DOI] [PubMed] [Google Scholar]
- 23.Haag S, Nandurkar S, Talley NJ. Regression of Barrett’s esophagus: the role of acid suppression, surgery, and ablative methods. Gastrointest Endosc. 1999;50:229–240. doi: 10.1016/s0016-5107(99)70230-0. [DOI] [PubMed] [Google Scholar]
- 24.Tobey N, Hosseini S, Argote C, et al. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. 2004;99:13–22. doi: 10.1046/j.1572-0241.2003.04018.x. [DOI] [PubMed] [Google Scholar]
- 25.Orlando R. Pathophysiolgoy of gastroesophageal reflux disease: Esophageal epithelial resistance. In: Castell DORJ, editor. The Esophagus. 4. Philadelphia: Williams & Wilkins; 2004. pp. 421–433. [Google Scholar]
- 26.Tobey NA, Carson JL, Alkiek RA, et al. Dilated intercellular spaces: A morphological feature of acid reflux–damaged human esophageal epithelium. Gastroenterology. 1996;111:1200–1205. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 27.Jovov B, Que J, Tobey NA, et al. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1039–1047. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvaro-Villegas J, Sobrino-Cossio S, Hernandez-Guerrero A, et al. Dilated intercellular spaces in subtypes of gastroesophagic reflux disease. Rev Esp Enferm Dig Organo Of Soc Esp Patol Dig. 2010;102:302–307. doi: 10.4321/s1130-01082010000500003. [DOI] [PubMed] [Google Scholar]
- 29.Moulton DE, Crandall W, Lakhani R, et al. Expression of a novel cadherin in the mouse and human intestine. Pediatr Res. 2004;55:927–934. doi: 10.1203/01.PDR.0000125260.46861.32. [DOI] [PubMed] [Google Scholar]
- 30.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 31.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 32.Guo X, Rao JN, Liu L, et al. Regulation of adherens junctions and epithelial paracellular permeability: A novel function for polyamines. Am J Physiol Cell Physiol. 2003;285:C1174–C1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 33.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta (BBA) Biomembr. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzog C, Haun RS, Ludwig A, et al. ADAM10 is the major sheddase responsible for the release of membrane-associated meprin A. J Biol Chem. 2014;289:13308–13322. doi: 10.1074/jbc.M114.559088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huovila A-PJ, Turner AJ, Pelto-Huikko M, et al. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Maretzky T, Reiss K, Ludwig A, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Practice & Research Clinical Gastroenterology. 2010;24:873–882. doi: 10.1016/j.bpg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahaleh M, Van Laethem J-L, Nagy N, et al. Long-term follow-up and factors predictive of recurrence in Barrett’s esophagus treated by argon plasma coagulation and acid suppression. Endoscopy. 2002;34:950–955. doi: 10.1055/s-2002-35847. [DOI] [PubMed] [Google Scholar]
- 39.Yeh R, Gerson L, Triadafilopoulos G. Efficacy of esomeprazole in controlling reflux symptoms, intraesophageal, and intragastric pH in patients with Barrett’s esophagus*. Dis Esophagus. 2003;16:193–198. doi: 10.1046/j.1442-2050.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- 40.Gerson L, Boparai V, Ullah N, et al. Oesophageal and gastric pH profiles in patients with gastro-oesophageal reflux disease and Barrett’s oesophagus treated with proton pump inhibitors. Aliment Pharmacol Ther. 2004;20:637–643. doi: 10.1111/j.1365-2036.2004.02127.x. [DOI] [PubMed] [Google Scholar]
- 41.Spechler SJ, Sharma P, Traxler B, et al. Gastric and esophageal pH in patients with Barrett’s esophagus treated with three esomeprazole dosages: a randomized, double-blind, crossover trial. Am J Gastroenterol. 2006;101:1964–1971. doi: 10.1111/j.1572-0241.2006.00661.x. [DOI] [PubMed] [Google Scholar]