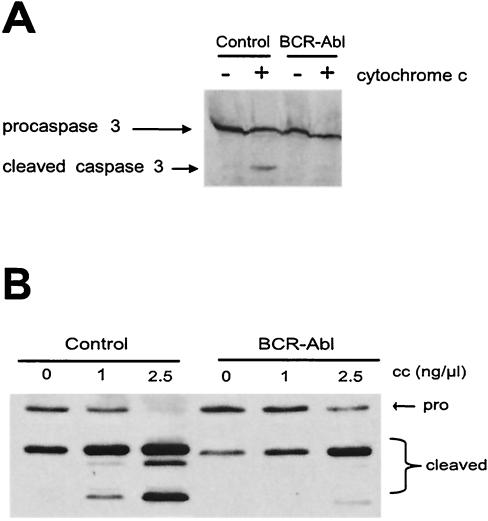
FIG. 3.
Bcr-Abl prevents processing of caspase 3 and caspase 9. (A) In vitro-translated, 35S-radiolabeled, catalytically inactive procaspase 3 was added to control GFP- or Bcr-Abl-expressing Rat-1 lysates in the presence of soluble cytochrome c (1 ng/μl). The processing of caspase 3 was observed via autoradiography. (B) Cell lysates from control GFP- or Bcr-Abl-expressing Rat-1 fibroblasts were incubated with 1 mM dATP and various concentrations of soluble cytochrome c. The processing of endogenous caspase 9 was assayed by Western analysis. Note that the fragment derived from caspase 9-mediated caspase 9 cleavage (the middle of the three cleaved bands indicative that the apoptosome has been activated) is barely visible in the presence of Bcr-Abl. The small amount of (unsuppressed) residual caspase 9 activity in this experiment most likely led to caspase 3 activation, resulting in amplification of caspase 9 processing through caspase 3-mediated caspase 9 cleavage (the upper band of the triplet).