Figure 7. Structure of the PDE4 MFD domain and candidate interaction site on MK2.
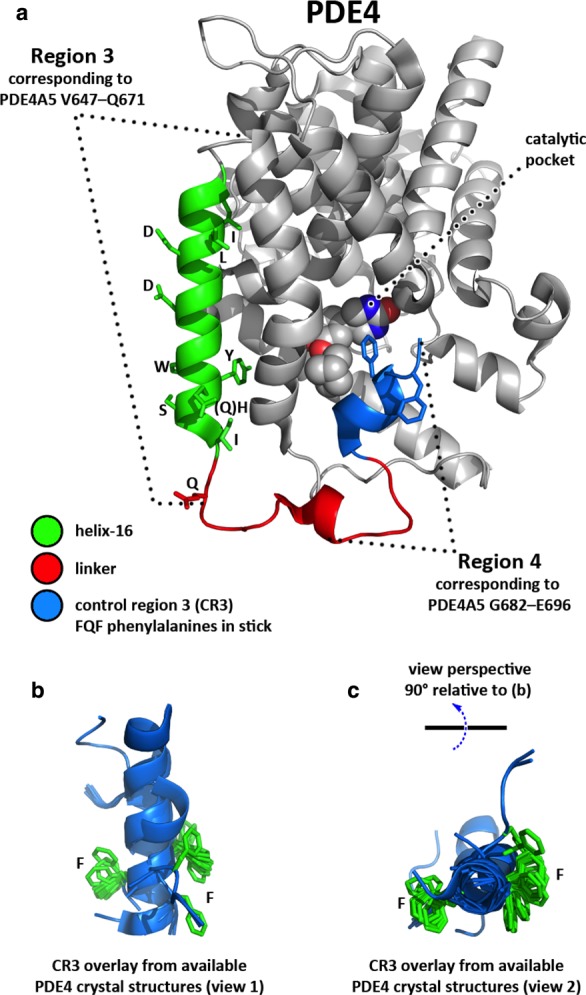
(a) The PDE4 core catalytic domain is shown from PDE4B co-crystal structure 3KKT with the bound inhibitor (spheres) marking the catalytic pocket. The Region 3–MK2 interaction sequence of PDE4A5 is shown mapped onto the structure, with specific interaction residues implicated in the array study shown (stick). The Region 4 peptide is similarly shown, spanning the C-terminal linker (red) and CR3 helix (blue), as far as the currently available crystallographic limit. The FQF phenylalanines are shown (blue stick) with CR3 in a capped position across the catalytic pocket. (b) In the majority of PDE4 crystal structures, the C-terminal linker and CR3 are disordered; an overlay of the CR3 peptides from those structures that exhibit some order reveals helical secondary structure but with a degree of conformational variability, especially affecting the presentation of the second Phe in the FQF motif (which lies at or near the limit of ordered structure determined to date). (c) An orthogonal view of the CR3 overlay is shown relative to that in (b).
