Abstract
Fragile X Syndrome (FXS) is a neurodevelopmental disorder caused by a trinucleotide (CGG) hyperexpansion in the FMR1 gene, functionally silencing transcription of the fragile x mental retardation protein (FMRP). This disorder is characterized by impaired cognition, communication, and social behavior. The purpose of this study was to investigate the development of ultrasonic vocalization (USV) behavior in a FMR1 deficient mouse model. On postnatal days (PD) 9–14, separate cohorts of FVB/NJ pups were removed from their home cage and isolation-induced USVs were recorded. There were significant genotype- and sex-dependent differences in USV behavior across the different testing days. FMR1 knockout mice showed a significant reduction in vocalizations across all days. There was also a significant difference in vocalizations between male and female mice. We found a significant decrease in total number of calls for KO males on PD9 and PD13, as well as an increase in the total number of calls for KO males on PD12. The KO males also had a significant increase in total call duration on PD12 and a reduction on PD13. The KO female had a significant decrease in the total number of calls on PD9 and PD10. They also had a significant decrease in the total call duration on PD9 and a marginal decrease in total call duration on PD10. These results provide additional evidence for communication deficits in FMR1 deficient mice and provide new insight suggesting sexually dimorphic vocalizations during the neonatal period.
Keywords: Neurodevelopmental disorder, fragile x syndrome, FXS, isolation-induced vocalizations, USV
Introduction
Fragile X Syndrome (FXS) is a neurodevelopmental disorder within the autism spectrum that is caused by a trinucleotide (CGG) hyperexpansion in the FMR1 gene, functionally silencing transcription of fragile x mental retardation protein (FMRP) [1]. This disorder is characterized by intellectual disability, as well as deficits in communication and other social behaviors. Mutations in the FMR1 gene are found in 2–6% of all individuals with ASD, making it the single largest genetic contributor to ASD. Furthermore, estimates of overlap suggest that between 25 and 50% of individuals with FXS meet DSM criteria for ASD [2]. For these reasons, the FMR1 knockout mouse is typically regarded as both a good model of the autistic phenotype and Fragile X Syndrome.
Neonatal crying behavior in mice has been characterized as ultrasonic whistle-like sounds occurring between 30–90kHz [3]. Neonatal mice are known to vocalize in response to isolation from the nest, dam, and littermates. Previous studies using this paradigm have shown alterations in neonatal vocalization behavior early in post-natal development in a variety of mouse models of ASD, including deletion of MECP2 [4] and SHANK1 [5]. Recent studies have shown that FMR1 deletion may alter ultrasonic vocalization (USV) behavior in mice on postnatal day (PD) 7 and 8 [6, 7].
It is well established that ASD has a higher prevalence in males than females. Similarly, prevalence estimates suggest that FXS occurs at a higher prevalence in males, with 1:5,000 males and 1:10,000 females being affected worldwide [8]. Given the X-linked inheritance pattern of FMR1 mutations, females are typically regarded as an “intermediate phenotype” and are not included in investigations. Therefore, monogenic models of ASD are rarely studied using both sexes, yielding few studies examining this interaction. Recent research in rat pups found clear dissimilarities between neonatal male and female calling behavior, which subsequently impacts maternal retrieval [9]. Previous analysis of other models of ASD has shown few sex-specific differences [10]. However, it is unknown whether sex-specific USV differences exist in FMR1 knockout mice. Therefore, the objective of this study will be to investigate genotype- and sex-specific differences in vocalization behavior in FMR1 knockout mice.
Materials & Methods
Subjects
For this study, male and female FVB/NJ mice were generated at Baylor University from a wildtype or knockout male and a heterozygous female for the FMR1 gene (n=305). All pups were designated into one of four groups: Knockout (KO) female (n=81), KO male (n=78), Wildtype (WT) female (n=71) and WT male (n=75). All pups were housed with both parents and littermates at an ambient temperature of 22°C and a 14-hour light and 10-hour dark diurnal cycle. All animals were given ad libitum access to food and water. All procedures involving mice were conducted in compliance with Baylor University Institutional Animal Care and Use Committee and the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Ultrasonic Vocalization Recording
Previous studies report that mouse pups emit ultrasonic vocalizations as a retrieval signal upon separation from their littermates, dam, and homecage environment. We chose to examine this isolation-induced vocalization behavior in order to characterize the vocal development in a mouse model of Fragile X-Syndrome (FXS). In order to control for the effects of habituation and contextual learning, separate pup cohorts were used for USV recordings on each day across postnatal days (PD) 9–14. Each pup was used for only one day of testing. Just prior to recordings all pups were placed in a housing pan with clean bedding, which was warmed with a heating pad to approximate ambient nesting temperature (≈ 35°C). During recordings all pups were placed individually into an acrylic, sound-attenuating chamber with USV detectors mounted in each of the four corners (Mini-3 Detector, Ultra Sound Advice, United Kingdom). Given that the majority of neonatal USVs occur within the 30–90kHz spectrum, recording microphones were set to 50, 60, 70, and 80-kHz in order to representatively sample vocalization behavior. Automatic detection software (Ultravox Software by Noldus, Netherlands) was used to measure both quantity and duration of calls at each measured call frequency during the 5-minute testing period. Following recordings pups were placed back into the warmed housing pan (≈ 35°C). Upon completion of all recordings, all pups were returned to their homecage. No more than 6 mice were tested during any recording session in order to limit total time spent away from the dam to 35 minutes or less.
Statistical Analyses
Neonatal USVs were first analyzed using a Multiple analysis of variance (MANOVA) with the independent factors of group, sex, day and frequency. The dependent variables were USVs quantity and total USVs duration. All data were analyzed using either GraphPad Prism 6 software (La Jolla, CA) or SPSS 20.0 (IBM, USA). Values are shown as mean ± S.E.M. for each group.
Results
Initial Analysis of Significant Main Effects and Interactions
We examined the number and duration of 50, 60, 70, and 80 kHz calls emitted by the pups across all days with a MANOVA. We used group, sex, day, and frequency as independent factors, and the number and duration of calls were the dependent measures. We found a main effect of group for number of calls F(1,1124) = 3.99, p < 0.05 and for duration of calls F(1,1124) = 5.22, p < 0.05. The WT mice produced more calls and for a longer duration, as compared to KO counterparts. There were main effects of sex for number of calls F(1,1124) = 20.62, p < 0.001 and for duration of calls F(1,1124) = 22.13, p < 0.001. In general, females emitted more calls and for a longer duration, as compared to male counterparts. There were main effects of day for number of calls F(5,1124) = 17.8, p < 0.001 and for duration of calls F(5,1124) = 12.7, p < 0.001. There were also a main effect of measured call frequency for number of calls F(3,1124) = 48.5, p < 0.001 and for duration of calls F(3,1124) = 149.6, p < 0.001. There were many interactions found with the MANOVA. The most important interaction was between group and day for number of calls F(5,1124) = 4.9, p < 0.001 and for duration of calls F(5,1124) = 3.1, p < 0.01. There was also a significant interaction between group x sex x day for number of calls F(5,1124) = 2.7, p < 0.05, but not for the duration of calls F(5,1124) = 1.9, p = 0.1.
Analyses of number of calls emitted per day for male and female subjects
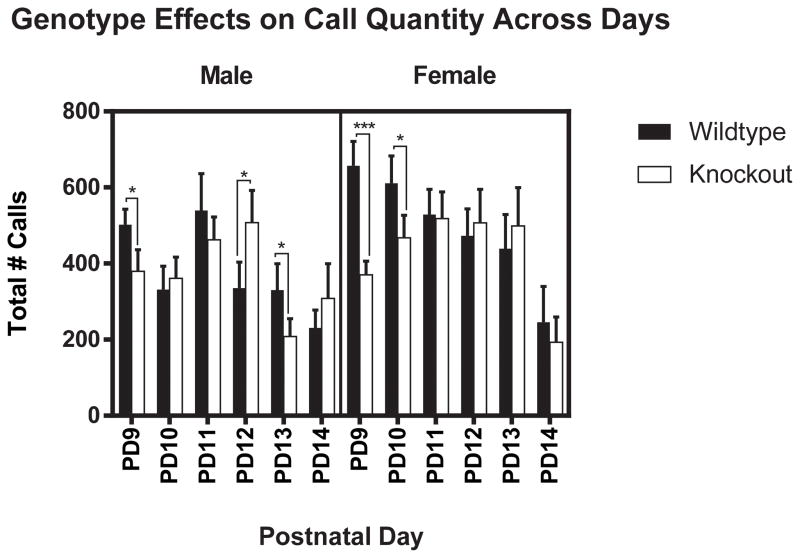
Due to the main effect of sex and the significant interactions we observed, we divided the analyses by sex then analyzed the number and duration of calls per measured call frequency across each day. On PD9, males displayed a genotype effect for the number of USVs F(1,88) = 5.9, p < 0.05, with FMR1 WT mice emitting more total USVs than KO mice (Fig. 1). Females also displayed a significant genotype effect F(1,92) = 24.3, p < 0.001, with FMR1 WT mice emitting more total USVs than KO mice (Fig. 1). On PD10, males did not display an effect of genotype F(1, 100) = 0.291, p = 0.60 (Fig. 1). There was also no observed interaction between genotype and measured call frequency F(3, 100) = 0.52, p =0.7 in male mice. In contrast, females did display genotype effects F(1,100) = 5.64, p = 0.020, with FMR1 WT mice emitting more total USVs than KO mice (Fig. 1). On PD11, neither males F(1,88) = 1.18, p = 0.28 (Fig. 1), nor females F(1, 88) = 0.02, p = 0.88 displayed an effect of genotype (Fig. 1). However, there was a marginal interaction between genotype and measured call frequency F(3, 88) = 2.63, p = 0.054 in female mice. We then performed follow-up independent t-tests to determine the specific differences between FMR1 WT and KO mice. We found that KO mice emitted fewer 80kHz vocalizations (M = 94.3± 28.6) compared to WT mice (M= 177.6 ± 22.0) t(1,46) = 2.7, p < 0.01. No other statistically significant differences were found for 50, 60, and 70 kHz. On PD12, males showed a significant genotype effect F(1,96) = 6.74, p < 0.05, with FMR1 KO mice emitting more total USVs than WT mice (Fig. 1). In contrast, females did not display an effect of genotype F(1, 96) = 0.232, p = 0.63 (Fig. 1). On PD13, males showed a significant genotype effect F(1,104) = 5.41, p < 0.05, with FMR1 WT mice emitting more total USVs than KO mice (Fig. 1). In contrast, females did not display an effect of genotype F(1, 96) = 0.46, p = 0.5 (Fig. 1). On PD14, males did not display an effect of genotype F(1,88) = 1.51, p = 0.22 on PD 14 (Fig. 1). There was also no observed interaction between genotype and measured call frequency F(3, 88) = 0.80, p = 0.5 in male mice. Females did not display an effect of genotype F(1, 96) = 0.69, p = 0.41 (Fig. 1).
Figure 1.
Genotype Effects on Call Quantity Across Days. Genotype had a significant effect on call quantity in males on PD9, 12, & 13, and in females on PD9 & 10. The bars represent the mean and the error bars represent the standard error of the mean. *p < 0.05;***p < 0.001.
Analyses of duration of calls emitted per day for male and female subjects
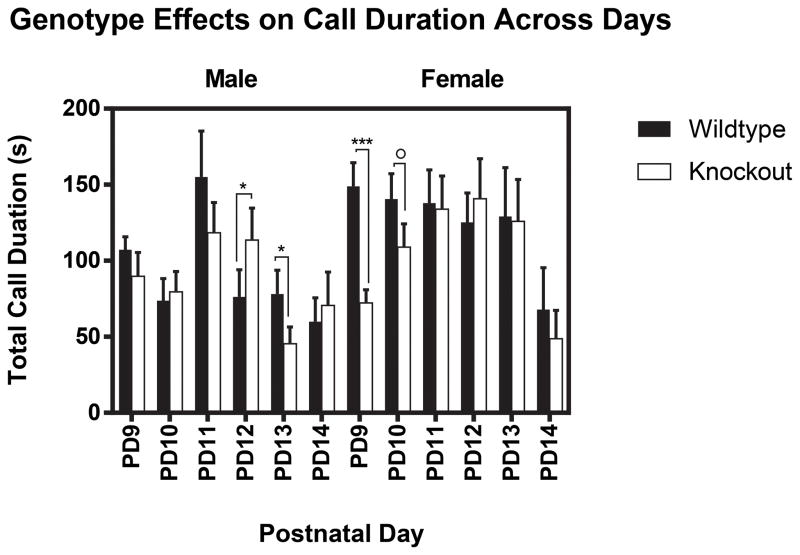
On PD9, males did not display an effect of genotype F(1, 88) = 1.92, p = 0.17 (Fig. 2). However, there was a significant interaction between genotype and measured call duration F(3, 88) = 2.90, p < 0.05. We then performed follow-up independent t-tests to determine the specific differences between FMR1 WT and KO mice. We found that male KO mice emitted 80kHz vocalizations for less time (M = 34.8 ± 7.74) compared to WT mice (M= 54.89 ± 5.34) t(1,22) = 2.1, p < 0.05. No other statistically significant differences were found for 50, 60, and 70 kHz. Contrasting from male counterparts, females displayed an effect of genotype F(1,92) = 24.85, p < 0.001, with female FMR1 WT mice vocalizing for greater total time than KO mice (p < 0.001) (Fig. 2). On PD10, males did not display an effect of genotype F(1,100) = 0.17, p = 0.68 (Fig. 2). Contrasting from male counterparts, females also displayed a marginally significant effect of genotype F(1,100) = 3.58, p = 0.06, with female FMR1 WT mice vocalizing for greater total time than KO mice (Fig. 2). On PD11, neither males F(1,88) = 2.2, p = 0.14, nor females F(1,88) = 0.01, p = 0.93 displayed an effect of genotype (Fig. 2). Interestingly, there was a marginal interaction between genotype and measured call duration F(3,88) = 2.63, p = 0.054 in female mice. Follow-up independent t-tests did not detect specific differences between FMR1 WT and KO mice. On PD12, males displayed an effect of genotype F(1,96) = 4.637, p < 0.05, with male FMR1 KO mice vocalizing for greater total time than WT mice (Fig. 2). Females did not display an effect of genotype F(1,96) = 0.58, p = 0.45 (Fig. 2). On PD13, males displayed an effect of genotype F(1,104) = 5.9, p < 0.05, with male FMR1 WT mice vocalizing for greater total time than KO mice (Fig. 2). Females did not display an effect of genotype F(1,88) = 0.01, p = 0.91 (Fig. 2). On PD14, neither males F(1,88) = 0.33, p = 0.57, nor females displayed an effect of genotype F(1,96) = 0.81, p = 0.37 (Fig. 2).
Figure 2.
Genotype Effects on Call Duration Across Days. Genotype had a significant effect on total call duration in males on PD12 & 13, and in females on PD9. Genotype had a marginally significant effect on total call duration in females on PD10. The bars represent the mean and the error bars represent the standard error of the mean. 0 = p = 0.05; *p < 0.05;***p < 0.001.
Discussion
The objective of the present study was to investigate genotype- and sex-specific differences in the USVs of FMR1 KO mice. There was a clear reduction in the frequency and duration of calls for KOs compared to WT across all days. There was also a significant difference in vocalizations between male and female mice. Due to sex differences and several statistical interactions, we analyzed the sexes separately. We found a significant decrease in total number of calls for KO males on PD9 and PD13, as well as an increase in total number of calls for KO males on PD12. The KO males also had a significant increase in total call duration on PD12 and a significant decrease on PD13. The female KOs had a significant decrease in the total number of calls on PD9 and PD10. The female KOs had a significant decrease in the total call duration on PD9 and a marginal decrease in total call duration on PD10.
Few pre-existing studies have investigated isolation-induced USVs in neonatal FMR1 KO mice. Our findings are complementary to previous work by Lai et al. (2014) who investigated spectral differences in FMR1 KO mice of FVB/NJ background strain [7]. The authors compared vocalization behavior between pups at the PD4, PD7, and PD10 time points. At the PD7 time point their findings show FMR1 KO pups emit a greater total number of calls. Interestingly, subsequent spectrographic analysis of PD7 USVs revealed that calling increases were specific to frequency jump calls. However, these authors did not observe any difference in USV duration on any measured postnatal day [7]. In contrast to both of these studies, a comprehensive spectral analysis of FMR1 KO USVs by Roy et al. (2012) found no quantitative changes in USV quantity or duration [6]. However, these authors observed that PD8 KO pups emitted a decreased proportion of downward calls, increased frequency range for complex calls, and increased carrier frequency for flat calls [6].
One significant difference between both of these studies and the present is that different developmental time points were used. Recording across PD9-14 allowed us to expand on previous findings and gain a more comprehensive perspective on the developmental trajectory of USV behavior. Previous studies have shown that calling rate follows an ontogenetic profile, which peaks at approximately PD8 and progressively falls until two to three weeks of age [11, 12]. However, we found that male KOs display increases in call quantity and duration on PD12, but decreases on PD9 and PD13. Taken together, we believe this may represent a genotype- and sex-dependent shift in the ontogenetic profile of USV development in FXS. Previous studies using the neonatal BTBR model of ASD have demonstrated similarly increased call quantity in mice [13]. Alternatively, given that USV development is subject to considerable strain-dependent variability, it is possible that the differences observed are due to the mouse strain used. [12, 14]. Future studies may investigate changes in vocalization behavior in several strains across the entirety of USV development in mice.
Deficits in social communication and language development have been highly characterized among children with FXS [15, 16]. Although no existing studies have investigated prelinguistic communicative behavior in human neonates FXS, our finding of significant sex-dependent differences in FMR1 KO communication behavior are in line with studies examining early childhood. In 2006, Brady et al. performed a descriptive and qualitative communication profile analysis in young children with FXS [15]. These authors found substantial differences in language development between sexes, as male children were completely nonverbal much longer (26.4 months) than female children (18.5 months).
Taken together, the results of this study find genotype- and sex-dependent effects on neonatal USVs. These findings support a growing body of evidence that neonatal vocalizations in mice may model early behavioral deficits across neurodevelopmental disorder. It is also among a limited number of investigations to give consideration to sex differences within FXS. Future directions will involve techniques to uncover spectral and temporal alterations in USV behavior. Both Roy et al. (2012) and Lai et al. (2014) have employed these techniques in the analysis of FXS USVs, yielding substantial progress in this area. However, these qualitative analyses have not yet been correlated with long-term behavioral phenotypic alterations. Studies identifying differences in call-type specific parameters could significantly enhance the predictive validity of neonatal USVs.
Acknowledgments
Grant Sponsor National Institute of Health (NIH); Grant Number: NS088776
Footnotes
Statement of Conflicts of Interest: None Declared
References
- 1.Verkerk AJ, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 2.Hagerman RJ, Rivera SM, Hagerman PJ. The fragile X family of disorders: A model for autism and targeted treatments. Current Pediatric Reviews. 2008;4(1):40–52. [Google Scholar]
- 3.Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125(1–2):49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- 4.Picker JD, et al. An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport. 2006;17(5):541–4. doi: 10.1097/01.wnr.0000208995.38695.2f. [DOI] [PubMed] [Google Scholar]
- 5.Wöhr M, et al. Communication Impairments in Mice Lacking Shank1: Reduced Levels of Ultrasonic Vocalizations and Scent Marking Behavior. PLoS ONE. 2011;6(6):e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy S, Watkins N, Heck D. Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One. 2012;7(9):e44816. doi: 10.1371/journal.pone.0044816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai JK, et al. Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development. Behav Brain Res. 2014;259:119–30. doi: 10.1016/j.bbr.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins M, et al. Preparation and validation of the first WHO international genetic reference panel for Fragile X syndrome. Eur J Hum Genet. 2011;19(1):10–7. doi: 10.1038/ejhg.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowers JM, et al. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2013;33(8):3276–83. doi: 10.1523/JNEUROSCI.0425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ey E, et al. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res. 2013;256:677–89. doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Elwood RW, Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev Psychobiol. 1982;15(3):221–7. doi: 10.1002/dev.420150306. [DOI] [PubMed] [Google Scholar]
- 12.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neuroscience and biobehavioral reviews. 2009;33(4):508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scattoni ML, et al. Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism. PLoS ONE. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roubertoux PL, et al. Vocalizations in newborn mice: genetic analysis. Behav Genet. 1996;26(4):427–37. doi: 10.1007/BF02359487. [DOI] [PubMed] [Google Scholar]
- 15.Brady N, et al. Communication in young children with fragile X syndrome: a qualitative study of mothers’ perspectives. Am J Speech Lang Pathol. 2006;15(4):353–64. doi: 10.1044/1058-0360(2006/033). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finestack LH, Richmond EK, Abbeduto L. Language Development in Individuals with Fragile X Syndrome. Topics in language disorders. 2009;29(2):133–148. doi: 10.1097/tld.0b013e3181a72016. [DOI] [PMC free article] [PubMed] [Google Scholar]