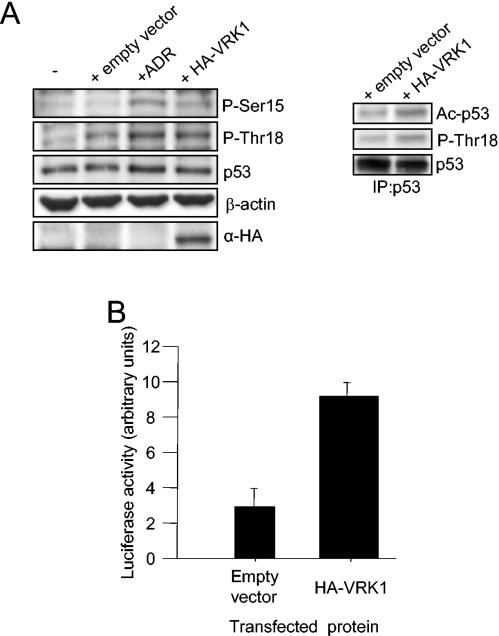
FIG. 7.
(A) VRK1 induces endogenous p53 phosphorylation and acetylation. A549 cells were transfected either with 4 μg of pCEFL-KZ plasmid alone or with HA-VRK1 construct and subjected to Western blotting with specific antibodies to detect p53 phosphorylated in Thr18, Ser15, or total protein. Untransfected cells and adriamycin (ADR)-treated cells are shown as controls (left). Total p53 protein was then immunoprecipitated (IP) and subjected to Western blotting to detect the acetylated form of the protein (right). (B) VRK1 overexpression enhances p53-dependent transcription. Cells were cotransfected with HA-VRK1 and a Bax reporter plasmid containing p53 response elements. Reporter luciferase activity was normalized for transfection levels with Renilla luciferase. The error bars represent standard deviations.