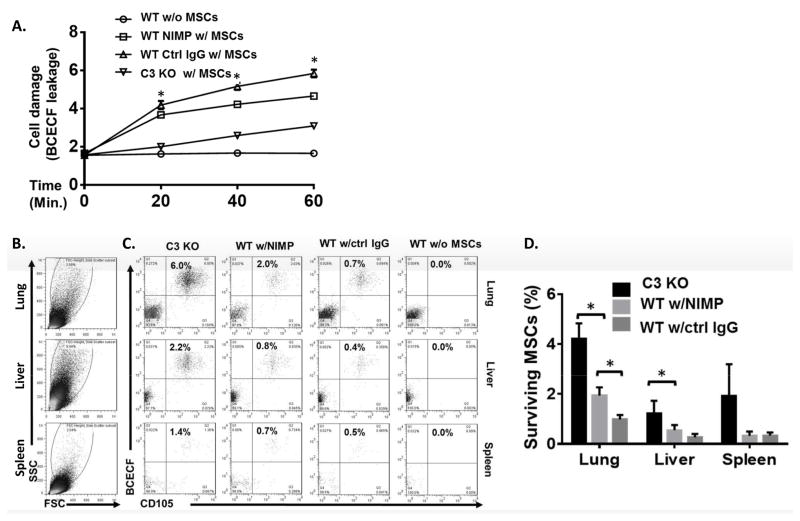
Figure 2. MSCs are damaged by complement-activated neutrophils after administration.
MSCs (1×106) were labeled with BCECF-AM and infused into WT mice depleted of neutrophils using anti-NIMP Ab (WT NIMP w/MSCs), WT controls not depleted of neutrophils (WT Ctrl IgG w/MSCs), and C3 KO mice (C3 KO w/MSCs). WT mice without MSC infusion (WT w/o MSCs) were included as controls. (A) At 0, 20, 40 and 60 min, blood was collected and levels of BCECF released from damaged MSCs were measured. Two-way ANOVA tests showed a significant difference between each group, * p<0.05. (B–D) Six hours after MSC infusion, the lung, liver, and spleen were collected and single cell suspensions prepared and incubated with Cy5.5-labeled anti-human CD105 mAb, a marker of human MSCs, followed by flow cytometric analysis. (B) shows large and living cells analyzed in C and D. (C and D) The large and living cells were then analyzed for the presence of surviving MSCs (BCECF+ CD105+); (C) shows representative results for surviving MSCs and (D) shows the combined results for all mice studied. n=4 in each group. One-way ANOVA and Tukey post-hoc test were used for data analysis, * p<0.05