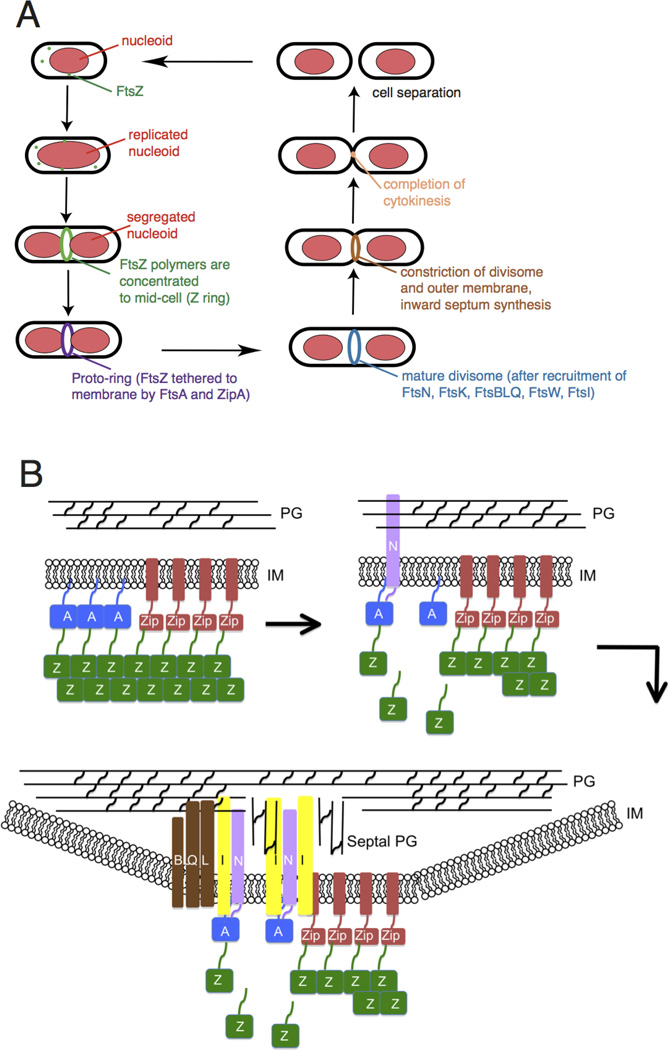
Fig. 1.
Overview of bacterial cytokinesis. (A) After E. coli cells replicate and segregate their chromosomes, organized as nucleoids, FtsZ and its membrane tethers are concentrated at midcell and organize into a Z ring or proto-ring. After recruitment of additional proteins, the proto-ring progresses into a mature divisome, which coordinates constriction of the inner and outer membranes with targeted cell wall hydrolases and ingrowing septal peptidoglycan. The ultimate result is separation into two equal-sized daughter cells. (B) ZipA and FtsA tether FtsZ protofilaments and polymer bundles to the membrane using flexible linkers and either a transmembrane segment (ZipA, red rectangle) or an amphipathic helix (FtsA). ZipA and FtsA are depicted as clustered, but they may be interspersed instead. In a later stage, ZipA bundling of FtsZ protofilaments is offset by FtsA-mediated disassembly. In addition, FtsA recruits FtsN via its cytoplasmic domain, which in turn may disrupt FtsA oligomers. FtsN’s periplasmic domain is targeted to peptidoglycan, reinforcing its localization and potentially stimulating FtsI and septal peptidoglycan synthesis. After septal peptidoglycan synthesis is underway, the other divisome proteins including the FtsBLQ complex transduce signals from the periplasm to the Z ring via FtsA, coordinating Z ring constriction (and FtsZ turnover) with septal peptidoglycan synthesis and targeted cell wall hydrolysis. For simplicity, many of the divisome proteins are not shown here, but they are shown in Fig. 4A.